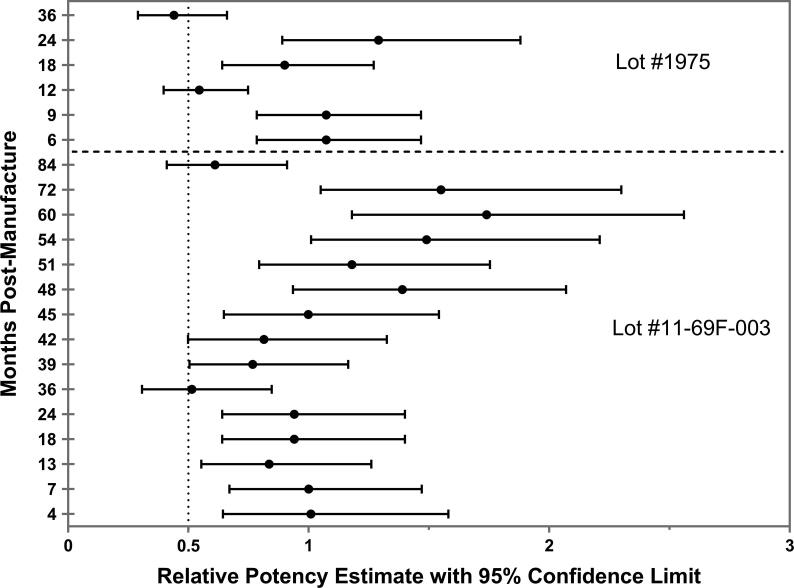
Fig. 1.
Estimates of relative potency using a compliance model at 84 and 36 months post-release for the first (#11-69F-003) and second (#1975) clinical lots, respectively.Sm-TSP-2 vaccine, which is at a concentration of 0.1 mg/mL Sm-TSP-2 with 0.8 mg/mL of Alhydrogel® in a sucrose/imidazole/Phosphate buffer (15% sucrose, 10 mM imidazole, 2 mM Phosphate, pH 7.4), was manufactured under current Good Manufacturing Practice (cGMP) conditions and stored in temperature-monitored refrigerators at 2–8 °C. The X-axis represents the relative potency estimates in black solid circle and its 95% confidence limits in black error bars. The Y-axis represents the testing time points in months post-manufacture. The vertical dotted line at 0.5 represents the specification for acceptance that the upper 95% confidence limit of the RP should not be less than 0.50.