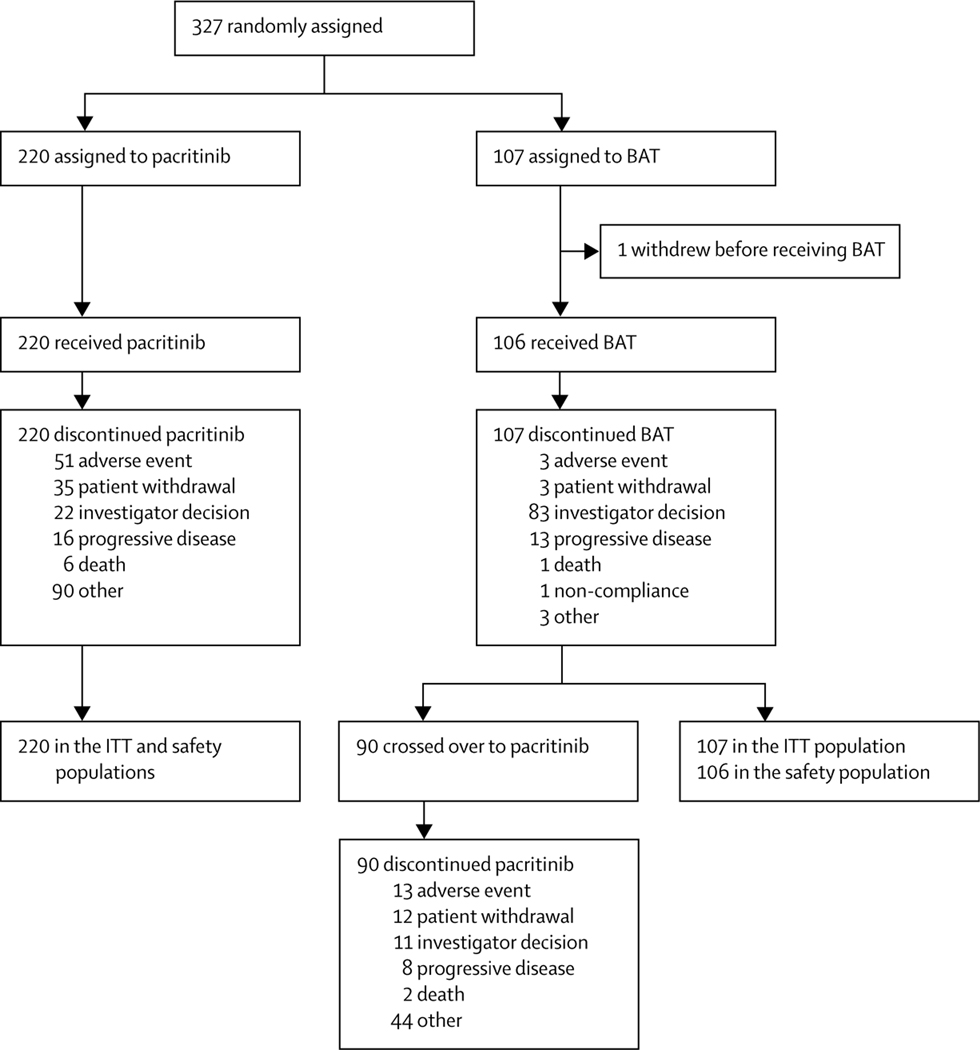
Figure 1: Trial profile.
84 patients who discontinued pacritinib for other reasons did so due to the clinical hold placed by the US Food and Drug Administration in February, 2016, (hold removed in January, 2017). Reasons given for the remaining six patients were incorrect diagnosis at study entry, treatment lock of efficacy, splenectomy, patient withdrawal from treatment but not from follow-up, clinical deterioration, and terminal illness leading to withdrawal of study drug and replacement with palliative care. 77 patients who discontinued BAT due to investigator decision did so to cross over to pacritinib treatment. The remaining six patients who discontinued BAT due to investigator decision were withdrawn because of the treating physician’s decision to change treatment (this patient remained on study for the following 3 months for safety follow-up), worsening of myelofibrosis symptoms, worsening status of patient (this patient died 1 week after discontinuing BAT), clinical progression (n=2; one patient remained on study for safety follow-up for 6 months and the other for 10 months; both patients died), and heavy overall complications caused by BAT. Of the remaining 13 patients who crossed over to pacritinib, 11 patients discontinued BAT because of disease progression, one patient discontinued BAT because of adverse events, and one patient because of patient withdrawal. BAT=best available therapy. ITT=intention-to-treat.