Abstract
Background
Social containment measures imposed in Europe during the lockdown to face COVID‐19 pandemic can generate long‐term potential threats for metabolic health.
Methods
A cohort of 494 non‐COVID‐19 subjects living in 21 EU countries were interviewed by an anonymous questionnaire exploring anthropometric and lifestyle changes during 1‐month lockdown. A subgroup of 41 overweight/obese Italian subjects with previously diagnosed nonalcoholic fatty liver (NAFLD) joined the study following a 12‐month follow‐up period promoting weight loss by healthy lifestyle.
Results
During the lockdown, body weight increased in 55% of subjects (average 2.4 ± 0.9 kg). Weight change increased with age, but not baseline body mass index. Subjects living in Italy had greater weight gain than those living in other European Countries. Weight gain during the lockdown was highest in subjects reporting no physical activity, and low adherence to Mediterranean diet. In the NAFLD group, weight gain occurred in 70% of cases. Subjects reporting weight loss during lockdown had decreased fatty liver score at 3 months before the lockdown, as compared with 1 year before.
Conclusions
Strict measures of social containment—even short‐term—pave the way to the increased risk of metabolic abnormalities in the medium‐long term. In this context, adherence to Mediterranean diet and regular physical activity play a protective role both in terms of weight gain and fatty liver development/progression, with implication for primary and secondary prevention. When adopting measures imposing social containment, intensive educational campaigns must increase public awareness about beneficial effects of healthy lifestyles.
Keywords: bariatric surgery, body mass index, COVID‐19 pandemic, lifestyles, lockdown, Mediterranean diet, metabolic syndrome, NAFLD, nonalcoholic fatty liver disease, obesity, physical activity, weight gain
1. INTRODUCTION
By 31 December 2020, the World Health Organization reports 81.475.053 confirmed cases of COVID‐19, including 1 798 050 deaths (https://covid19.who.int/). Europe is the second most affected continent after Americas, with about 26 million cases and 576 721 COVID‐related deaths. 1
After the declaration of the pandemic by WHO (March 11, 2020), 2 several governments imposed the lockdown as a containment measure and the aim of potentially limiting and reversing the infection spread. During the lockdown, people were not allowed to leave homes, apart from buying groceries and emergency medicines. As a result of this intense social isolation, people suffered from stress, frustrations due to financial losses, inadequate supplies and fear of COVID‐19 infection this negative feelings caused among population created an unhealthy environment to survive. 3 , 4 , 5 Thus, besides evident beneficial effects on mortality and morbidity rates, 6 the lockdown generated major economic negative effects, 7 as well as increased risk of anxiety, and depression. 8
In addition, the chance of developing an increased risk of unhealthy lifestyles increased, mainly due to sedentary behaviours, low adherence to a healthy diet and modifications in smoking and sleeping habits. The interruption of the routine work life generated boredom, which in turn has been reported to contribute to higher energy intake. 9 In addition, smart working and digital education likely lead to decreased physical activity, less energy expenditure and possible body weight gain.
The combination of such unexpected and novel lifestyles likely impact subjects exposed to elevated cardio‐metabolic risk and those pursuing a weight loss programme.
Obesity and related metabolic conditions, including diabetes, dyslipidemia, hypertension often combined within the metabolic syndrome are strong risk factors for nonalcoholic fatty liver disease (NAFLD), the ‘fellow traveller’ with the metabolic syndrome. 10 , 11 , 12 , 13 NAFLD is the most common cause of chronic liver disease worldwide, 14 linked with increased risk for advanced liver disease, liver‐related mortality 15 and nonliver‐related complications, such as cardiovascular disease and malignancy. 16 , 17 Thus, the high prevalence of NAFLD (about 25% of the adult population, increasing to 76% in type 2 diabetes mellitus 18 ) and its related health risks justify all efforts aimed at primary and secondary prevention. From this point of view, unhealthy lifestyles potentially caused by strict measures of social containment can interrupt and compromise the beneficial effects of prevention measures in high‐risk patients.
We aimed to investigate how the intense social isolation imposed by the lockdown could affect lifestyles among EU citizens, potentially leading to public health threats. In addition, we evaluated possible negative effects of the lockdown in subjects with NAFLD previously enrolled in a monitoring programme oriented at obtaining weight loss by healthy lifestyle.
2. SUBJECTS AND METHODS
A cohort of 494 European subjects joined the anonymous survey (European group).
Within this cohort, a subgroup consisted of 41 NAFLD Italian subjects who had joined the ‘Foie Gras’ Horizon 2020 project enrolled at the outpatient clinic (Division of Internal Medicine of the large Regional Hospital ‘Policlinico’, in Bari, Italy) (NAFLD Group).
The NAFLD group consisted of 41 overweight or obese subjects with metabolic abnormalities and liver steatosis, as assessed by abdominal ultrasonography (see below). Main causes of chronic liver disease (viral, alcoholic, drug‐induced damage, autoimmune diseases) were carefully excluded after history, physical examination and blood samples for the determination of hepatitis B/C viral markers and autoantibodies. All subjects entered an educational follow‐up programme for weight loss starting 1 year before the lockdown. They had been adequately trained on beneficial effects of healthy diet and daily physical activity. Subjects in this subgroup also underwent, 1 year (basal) and 3 months before the lockdown (T1), anthropometric evaluation and measurement of liver steatosis. Liver steatosis was assessed with the Noblus‐E ultrasonographic equipment (Hitachi Medical) using a convex multifrequency (3.5‐12.0 MHz) probe according to a well‐standardized protocol, previously described by our group. 19 , 20 In brief, in the fasting subject, the echogenicity of liver parenchyma was compared against the kidney cortex echogenicity as the control parenchyma and defined as either isoechoic (normal liver) or hyperechoic = ‘bright’ parenchyma (steatotic liver). A bright liver is a sensitive marker of liver steatosis, although its accuracy is poor for mild steatosis (<30%) and for the detection of underlying inflammation. 21 Liver steatosis was graded as grade 0: absence of fatty liver; grade 1: minor increase in liver echogenicity; and grade 2: marked increase in liver echogenicity with poor penetration of posterior segment from the right lobe, poor or any visual images from the hepatic vessels and diaphragm. 20
All subjects who participated in the general survey filled an anonymous questionnaire on a voluntary basis. For this, no personal information was collected, and written consent was not required. At the beginning of the questionnaire, a specific paragraph informed about the aim of the research (see below). Permission served to use the collected data for analysis and publication.
The period referring to data collection ranged from 15 April to 15 May 2020, when almost every European country had imposed the lockdown. European adults were encouraged to participate in an online, observational study, completing an online questionnaire created using Google forms tool. The questionnaire was distributed through snowball sampling method, using social media (Facebook, WhatsApp, LinkedIn) and e‐mails. Inclusion criteria were age >18 years old, and without current or previous COVID‐19 symptoms or infection. The self‐administered questionnaire contained 33 questions divided into four subsections, that is,
socio‐demographic information: gender, age, nationality, level of education, occupational status, place of residence and living situation at home;
anthropometric information: height (as metres) and body weight (as kg before and after lockdown);
-
dietary information, including:
frequency of food consumption and adherence to Mediterranean diet (see below);
structured questionnaire exploring junk food consumption, the number of meals consumed per day before and after/during lockdown and intake of nutritional supplements;
-
Sleeping time (ie number of sleep hours per day), alcohol consumption (ie number of alcohol units consumed per day), smoking (daily number of cigarettes), and physical activity during the lockdown (ie presence of regular exercise during the lockdown, and weekly frequency of moderate/vigorous physical activity). According to presence/absence and extent/timing of physical activity, enrolled subjects were thereafter classified in the following subgroups:
no physical activity (low level);
physical activity between 10 minutes and 2.5 hours/week (moderate level);
physical activity for more than 2.5 hours/week (high levels).
The body mass index (BMI, Quetelet's index) was calculated from the weight and square of the height according to the following formula: BMI = body weight (in kg) ÷ height (in metres) squared. BMI was further stratified according to the National Institutes of Health (NIH) and World Health Organization (WHO) 22 , 23 as underweight (BMI <18.5 kg/m2), normal weight (BMI ≥18.5‐24.9 kg/m2), overweight (BMI ≥25.0‐29.9 kg/m2) and obesity (BMI ≥30 kg/m2).
The adherence to Mediterranean Diet was calculated on a 18‐point scale score ranging from 0 (min) to 18 (max). 24 For this, nine food products were grouped according to:
Items generally consumed in prevalent quantities (ie vegetables, fruits, legumes, cereals, fish and extra‐virgin olive oil (EVOO)). We attributed 2 points as the highest category of consumption, 1 point for the middle and 0 to lowest category.
Items of not prevalent consumption (meat and meat products, dairy products, alcohol and junk foods). In this case, we attributed 2 points for the lowest/none category, 1 point for the middle category and 0 point for the highest category of consumption.
The final score was the sum of individual scores and indicated adherence to Mediterranean diet. A value ranging from 0 to 6 indicated low adherence, a value ranging from 7 to 12 indicated moderate adherence, and a value ranging from 13 to 18 indicated higher adherence.
After 1‐month from entry, body weight was considered either increased (≥1 kg from baseline), decreased (≤1 kg from baseline) or stable (maximum weight decrease or increase of 0.99 kg).
2.1. Ethical aspects
The general questionnaire survey was approved by Ethics committee, University of Bari ‘Aldo Moro’ (study number 6672 protocol number 0009048).
The study protocol which explored subjects in the NAFLD subgroup was part of the Foie Gras project (‘Foie Gras’ Horizon 2020 project). This protocol was approved by the local Ethics Committee (study number 5408, protocol number 0013869; AOUCPG23/COMET/P). Before this study, all subjects in the NAFLD subgroup gave full written informed consent to allow all authors to access and use the data for research purposes.
2.2. Statistical analysis
Data are expressed as mean and standard error (SE) for continuous variables. Frequency and percentages were used for categorical variables. The chi‐square test (proportions), the Mann‐Whitney U test (unpaired data), the Wilcoxon signed‐rank test (paired data) or the Kruskal‐Wallis multiple comparison Z‐value test (inter‐group differences) were employed to evaluate intra‐ or inter‐group differences. The Spearman correlation coefficient estimated the correlations. All statistical analyses were performed using NCSS software, and statistical significance was declared if a two‐sided P‐value was <.05. 25 , 26 Graphs were constructed with the SigmaPlot v. 14.05 version (Systat Software, Inc). Reporting of the study conforms to broad EQUATOR guidelines.
3. RESULTS
3.1. European group
The anthropometric features of the study population appear in Table 1.
TABLE 1.
Anthropometric features of the study population
| All | Females | Males | P * | |
|---|---|---|---|---|
| Number (%) | 494 (100%) | 303 (61.3%) | 191 (38.7%) | .0001 |
| Age (y) | 33.4 ± 0.58 | 32.5 ± 0.7 | 34.8 ± 1.0 | .02 |
| Age class, n. (%) | ||||
| 18‐30 y | 309 (62.5%) | 198 (65.3%) | 111 (58.4%) | n.s. |
| 31‐60 y | 159 (32.2%) | 95 (31.4%) | 63 (33.2%) | n.s. |
| >60 y | 26 (5.3%) | 10 (3.3%) | 16 (8.4%) | n.s. |
| BMI (kg/m2) | 23.6 ± 0.2 | 23.0 ± 0.3 | 24.6 ± 0.3 | n.s. |
| Normal weight (%) | 355 (71.9%) | 229 (75.6%) | 125 (65.4%) | n.s. |
| Overweight (%) | 96 (19.4%) | 51 (16.8%) | 45 (23.6%) | n.s. |
| Obese (%) | 43 (8.7%) | 23 (7.6%) | 20 (10.5%) | n.s. |
Difference between proportions evaluated by chi‐square test.
Abbreviations: BMI, body mass index; n.s., not significant.
Between gender; data expressed as mean ±standard error.
Females were more willing to answer than males (61.3% vs. 38.7%). Mean age of the whole group was 33.4 ± 0.58 years, and males were slightly older than females. The distribution of subjects decreased progressively with age (62.5%, 32.2%, 5.3% with age 18‐30, 31‐60 and >60 years, respectively) and was comparable between genders. Mean BMI of the whole cohort was 23.6 ± 0.2 kg/m2 at baseline and comparable between genders. Normal weight subjects were more represented (71.9%) than overweight and obese subjects.
The socio‐demographic characteristics of participants appear in Table 2.
TABLE 2.
Socio‐demographic characteristics of participants
|
All (N = 494) |
Females (N = 303) |
Males (N = 191) |
P * | |
|---|---|---|---|---|
| Geographical area | ||||
| Italian residents | 376 (76.1%) | 226 (60.1%) | 150 (78.5%) | .02 |
| Other European residents | 118 (23.9%) | 77 (65.3%) | 41 (21.5%) | .02 |
| Education level | ||||
| Primary school | 18 (3.6%) | 11 (3.6%) | 7 (3.7%) | n.s. |
| High school | 193 (39.0%) | 118 (38.9) | 75 (39.3%) | n.s. |
| University graduate | 178 (36.0%) | 108 (35.6%) | 70 (36.6%) | n.s. |
| Postgraduate | 105 (21.3%) | 66 (21.8%) | 39 (20.4%) | n.s. |
| Employment status | ||||
| Full‐time work | 206 (41.7%) | 112 (37.0%) | 94 (49.2%) | .0043 |
| Students | 181 (36.6%) | 120 (39.6%) | 61 (31.9%) | .0361 |
| Unemployed | 44 (8.9%) | 26 (8.6%) | 18 (9.4%) | n.s. |
| Retiree | 8 (1.6%) | 3 (1%) | 5 (2.6%) | n.s. |
| Other | 55 (11.1%) | 42 (13.9%) | 13 (6.8%) | .039 |
| Residence | ||||
| Outskirts of city | 208 (42.1%) | 127 (41.9%) | 81 (42.4%) | n.s. |
| City centre | 234 (47.4%) | 140 (46.2%) | 94 (49.2%) | n.s. |
| Countryside | 52 (10.5%) | 36 (11.9%) | 16 (8.4%) | n.s. |
| Living conditions during lockdown at home | ||||
| With family | 368 (74.5%) | 225 (74.3%) | 143 (74.9%) | n.s. |
| Alone | 45 (9.1%) | 28 (9.2%) | 18 (9.4%) | n.s. |
| Friends | 17 (3.4%) | 10 (3.3%) | 7 (3.7%) | n.s. |
| Couples | 54 (10.9%) | 36 (11.9%) | 18 (9.4%) | n.s. |
| Other | 9 (1.8%) | 4 (1.3%) | 5 (2.6%) | n.s. |
Data are expressed as number and percentages.
Abbreviation: n.s., not significant.
Comparison between gender; difference evaluated by chi‐square test.
The majority of enrolled subjects (considering both sex) were from Italy (76.1% Italians vs. 23.9% residents in other European countries, P = .0001). In the majority of cases, enrolled subjects had high school education, irrespective of gender and were full‐time workers or students. Enrolled subjects living in countryside were less than those living in urban centres (city centre/outskirts). Participants declared in the majority of cases to live with their families, irrespective of gender.
3.1.1. Effect of lockdown
After lockdown, 273 subjects (55.2%) reported an increased body weight consisting of an average increment of 2.4 ± 0.9 kg, as compared with weight measured before the lockdown. A minority of subjects (n = 107, 21.7%) reported a decreased body weight (on average −2.4 ± 0.1 kg). In the remaining subjects (n = 134, 27.1%), the weight remained stable during the lockdown.
Subjects, who accumulated body weight, also reported an increased number of daily meals during lockdown (2.2 ± 0.04 vs 1.9 ± 0.05 before the lockdown, P = .001, Wilcoxon signed‐rank test). This was not the case in individuals with unchanged (2.1 ± 0.07 meals/day both before and during the lockdown) or decreased body weight (2.2 ± 0.08, vs 2.2 ± 0.07 meals/day before the lockdown, P = NS).
In the whole population, the weight variation during the lockdown was influenced by education, with increased weight gain in subjects with the lowest education level (primary school, on average +4.3 ± 0.8 kg), as compared to those who completed high schools (+1.3 ± 0.2 kg), were university graduate (+0.8 ± 0.2 kg) or postgraduate (0.4 ± 0.3 kg, P = .000007, Kruskal‐Wallis multiple comparison Z‐value test). When place of residence was considered, subjects living in city centre (+1.26 ± 0.2 kg) and outskirts of city (+1.14 ± 0.2 kg) showed a higher weight gain, as compared with those living in countryside (−0.1 ± 0.4 kg, P = .004, Kruskal‐Wallis multiple comparison Z‐value test). A comparable weight variation was recorded when subjects were stratified according to employment status, living conditions during lockdown at home (P=NS, data not shown) and alcohol consumption (Table 4). The majority of enrolled subjects were no smokers (n = 398, 80.6%), and the average weight gain during the lockdown was comparable in these subjects (1.04 ± 0.1 kg) and in those who smoked 1‐5 (0.7 ± 0.5 kg), 5‐10 (1.9 ± 0.6 kg), 10‐15 (0.2 ± 0.9 kg) or more than 15 cigarettes/day (2.3 ± 1 kg, P = NS).
TABLE 4.
Individual items of the Mediterranean diet (score for each food product) in subjects with decreased, unchanged or increased body weight during the lockdown
| Food products | Decreased Weight (n = 107) | Unchanged Weight (n = 134) | Increased Weight (n = 273) | P |
|---|---|---|---|---|
| Fruit (portions/d) | 0.9 ± 0.06 | 1.02 ± 0.06* | 0.8 ± 0.04 | .03 |
| Vegetables (portions/d) | 1.1 ± 0.06* | 0.9 ± 0.05* | 0.7 ± 0.03 | .0001 |
| Legumes (portions/wk) | 1.01 ± 0.06* | 0.8 ± 0.05 | 0.7 ± 0.04 | .03 |
| Cereal (portions/d) | 0.5 ± 0.06 | 0.6 ± 0.05 | 0.5 ± 0.04 | n.s. |
| Fish (portions/wk) | 0.8 ± 0.06 | 0.7 ± 0.05 | 0.6 ± 0.03 | n.s. |
| Meat and meat products (portions/d) | 0.8 ± 0.06 | 0.7 ± 0.06 | 0.8 ± 0.04 | n.s. |
| Dairy & dairy products (portions/d) | 0.7 ± 0.06 | 0.6 ± 0.05 | 0.5 ± 0.04 | n.s. |
| Alcohol (units/d) | 1.7 ± 0.04 | 1.6 ± 0.04 | 1.7 ± 0.02 | n.s. |
| Extra‐virgin olive oil (EVOO, portions/d) | 1.3 ± 0.07 | 1.2 ± 0.06 | 1.3 ± 0.04 | n.s. |
| Junk food (portions/d) | 1.9 ± 0.04* | 1.7 ± 0.04 | 1.7 ± 0.03 | .007 |
Data are mean ± standard error; * P < .04 vs. increased weight. Difference was evaluated by Kruskal‐Wallis multiple comparison Z‐value test.
Fruits, 1 portion = 100 grams; vegetables, 1 portion = 100 grams; legumes, 1 portion = 70 grams; cereals, 1 portion = 130 grams; fish, 1 portion = 100 grams; meat and meat products, 1 portion = 80 grams; dairy and dairy products, 1 portion = 180 grams; alcohol, 1 unit = 12 grams; EVOO, 1 portion = 14 grams; junk food, 1 portion = 100 grams. 66
The number of sleep hours per night was similar in subjects with a decreased (7.5 ± 0.1 hours), unchanged (7.3 ± 0.1 hours) or increased body weight (7.2 ± 0.07 hours).
Table 3 shows the average weight gain after 1‐month lockdown in the whole population according to gender, age distribution, BMI, country of residence, level of physical activity and adherence to Mediterranean diet. Males showed a significantly greater weight gain than females.
TABLE 3.
Effect of lockdown on weight gain according to gender, age class, BMI, country of residence, level of physical activity and dietary habits
| Participants characteristics | Number (%) | Weight gain (Kg) | P value within groups |
|---|---|---|---|
| Gender | |||
| Male | 191 (38.7%) | 1.0 ± 0.2 | .01 |
| Female | 303 (61.3%) | 0.5 ± 0.1 | |
| Age class | |||
| 18‐30 y | 309 (62.5%) | 0.5 ± 0.1 | *P < .05 vs 18‐30 y |
| 31‐60 y | 159 (32.2%) | 1.0 ± 0.2* | |
| >60 y | 26 (5.2%) | 0.8 ± 0.5 | |
| BMI group | |||
| Normal weight | 355 (71.9%) | 0.7 ± 0.1 | n.s. |
| Overweight | 96 (19.4%) | 0.9 ± 0.2 | |
| Obese | 43 (8.7%) | 0.2 ± 0.4 | |
| Country of residence | |||
| Italians | 376 (76.1%) | 0.9 ± 0.1 | .00001 |
| Other Europeans residents | 118 (23.9%) | ‐0.07 ± 0.2 | |
| Physical activity during the lockdown | |||
| No | 139 (28.1%) | 1.4 ± 0.2 | *P < .0003 vs no physical activity |
| Yes | 355 (71.9%) | 0.4 ± 0.1* | |
| Physical activity levels | |||
| None | 139 (28.1%) | 1.4 ± 0.2 |
*P < .05 vs no physical activity # P < .05 vs 10 min‐2.5 h/wk |
| 10 min‐2.5 h/wk | 198 (40.0%) | 0.6 ± 0.2* | |
| >2.5 h/wk | 157 (31.8%) | 0.2 ± 0.2*# | |
| Mediterranean diet score | |||
| Low adherence | 35 (7.1%) | 1.9 ± 0.4 | *P < .05 vs low adherence |
| Moderate adherence | 365 (73.9%) | 0.6 ± 0.1* | |
| High adherence | 94 (19.0%) | 0.5 ± 0.2* | |
Data are expressed as number and percentage for categorical variables and as mean ± standard error for continuous variables. Difference was evaluated by Mann‐Whitney U test or by Kruskal‐Wallis multiple comparison Z‐value test, as appropriate. *P, # P indicate intra‐subgroup significant differences.
A weak, positive correlation was present between weight change and age (P = .01; ρ = 0.11). When subjects were divided according to age, the weight gain was significantly higher in subjects aged 31‐60 years, as compared to younger subjects.
Weight changes did not correlate with the BMI values recorded before lockdown. No significant difference emerged when subjects were divided according to BMI.
On average, subjects living in Italy reported a greater weight gain, as compared with those living in other European Countries.
In the whole group of subjects, the weight gain was significantly higher in those who reported no physical activity during the lockdown, as compared to those who reported regular low or high levels of exercise. Weight gain was absent or minimal in both these two last subgroups and was the lowest in subjects reporting exercise for more than 2.5 hours/week.
Similarly, the highest weight gain was reported by subjects with low adherence to Mediterranean diet, as compared with those with moderate or high adherence. In particular, subjects with an increased weight during the lockdown had lower scores of consumptions of fruits, vegetables and legumes, as compared with those with a decreased or unchanged weight. Conversely, subjects with a decreased weight reported the highest daily consumption of junk food (Table 4).
Although the largest group of subjects were living in Italy and national subgroups were not representative of the whole country of origin, a different weight change was recorded in subjects from the more represented European countries (ie ≥10 enrolled subjects per country, Figure 1A). In particular, subjects living in Italy and Portugal tended to weight gain. Conversely, subjects living in Germany and Spain had, on average, a weight loss during the lockdown. As shown in Figure 1B, the mean weight change occurring in these subgroups during the lockdown was independent from the usual level of physical activity (ie percentage of subjects performing aerobic sport) recorded in the same countries, as reported by EUROSTAT (https://ec.europa.eu/eurostat/databrowser/view/hlth_ehis_pe3u$DV_468/default/table?lang=en, last update 11/11/2020).
FIGURE 1.
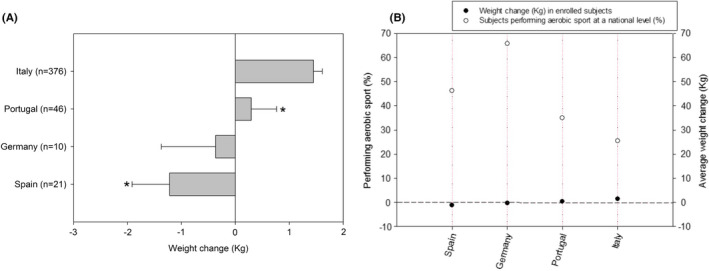
A, Weight changes (after vs. before lockdown) in non‐COVID‐19 subjects from main European countries represented (ie ≥10 enrolled subjects), stratified according to living country. The number of enrolled subjects for each country is indicated in parenthesis. Bars indicate means, vertical lines indicate SE. *P < .03 vs Italians (ANOVA followed Fishers' post hoc test). B, Average weight change (kg) observed in enrolled subjects from main European countries represented (ie ≥10 enrolled subjects) during the lockdown (filled circles) and level of physical activity (ie percentage of subjects performing aerobic sport, white circles) in the general population from the same countries, as reported by EUROSTAT (https://ec.europa.eu/eurostat/databrowser/view/hlth_ehis_pe3u$DV_468/default/table?lang=en, last update 11/11/2020)
As shown in Figure 2, the adherence score to Mediterranean diet was slightly increased in subjects from countries reporting weight loss, as compared to subjects living in countries reporting weight gain (11.2 ± 0.3 vs.10.1 ± 0.1, respectively, P = .042).
FIGURE 2.
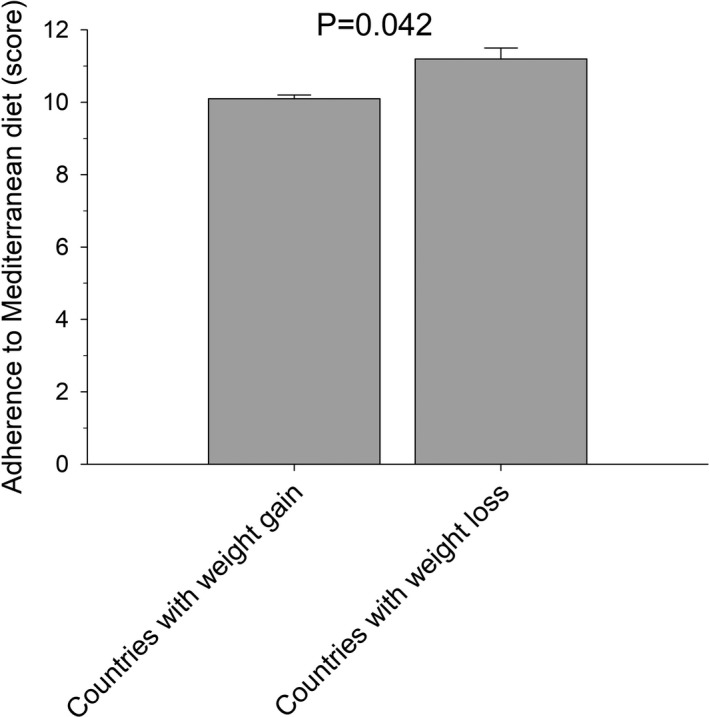
Adherence to Mediterranean diet (score) in subjects living in countries where, on average, a weight gain or a weight loss was recorded. Bars indicate means, vertical lines indicate SE. *P < .05, Student's t test for unpaired data)
3.2. NAFLD group
This group consisted of 41 overweight and obese subjects with NAFLD (24 males, 17 females; age 49.5 ± 1.9 years) already enrolled 1 year before lockdown. The protocol consisted of an educational programme aiming to improve healthy lifestyles, to promote weight loss and likely to ameliorate NAFLD. The COVID‐19 pandemic had an impact on this protocol. Indeed, during the lockdown, subjects reported weight gain in 70% of cases. Both body weight and BMI increased irrespective of gender (Table 5). As a consequence, mean weight gain after lockdown was 1.6 ± 0.6 kg (all), 1.9 ± 0.7 kg (males) and 1.1 ± 1.1 kg (females).
TABLE 5.
Effects of lockdown on weight and BMI in the NAFLD group a
| Before lockdown | After lockdown | P | |
|---|---|---|---|
| Weight (kg) | |||
| All | 89.7 ± 3.2 | 91.3 ± 3.3 | .0004 |
| Males | 93.7 ± 4.6 | 95.7 ± 4.7 | .009 |
| Females | 84.1 ± 4.2 | 85.2 ± 4.1 | .02 |
| BMI (kg/m2) | |||
| All | 30.5 ± 1.0 | 31.0 ± 1.0 | .0004 |
| Males | 29.6 ± 1.2 | 30.2 ± 5.8 | .01 |
| Females | 31.7 ± 1.7 | 32.1 ± 7.0 | .02 |
Data are mean ± standard error for continuous variables. Difference was evaluated by Mann‐Whitney U test (unpaired data) or Wilcoxon signed‐rank test (paired data).
The group consisted of 41 overweight and obese subjects with NAFLD enrolled 1 y before the lockdown into an educational follow‐up programme aiming at healthy lifestyles, weight loss and improved NAFLD.
Figure 3A shows the overall profile of mean body weight at enrolment (ie 12 months before the lockdown, 93.9 ± 3.2 kg), 3 months before the lockdown (91.6 ± 3.2 kg), at the start of lockdown (90.9 ± 3.2 kg) and at the end of lockdown (92.5 ± 3.2 kg). Notably, there was a trend to continuous decrement of body weight until the start of lockdown (−2.9 ± 1.5 kg compared baseline weight at enrolment). The lockdown, however, interrupted this trend and was associated with a novel increment of body weight (ie −1.4 ± 1.5 kg in the whole group, as compared baseline weight at enrolment).
FIGURE 3.
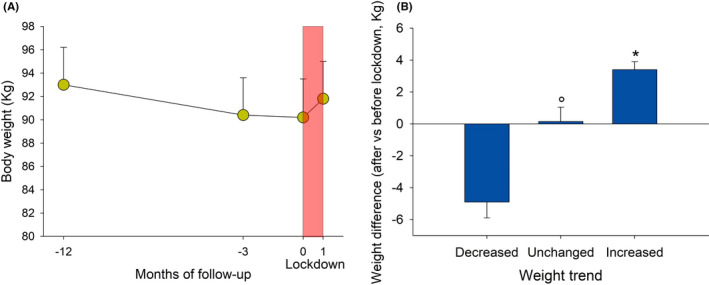
A, Body weight measured 1 y, 3 mo before the lockdown (LD), at the lockdown and 1 mo after the lockdown, in a subgroup of overweight/obese subjects previously enrolled in a monitoring programme for weight loss (NAFLD group). Subjects were adequately trained on beneficial effects of healthy diet and regular physical activity. Bars indicate means, and vertical lines indicate SE. B, Average weight change in subjects reporting decreased, unchanged and increased weight after the lockdown. *P < .05 vs decreased and unchanged weight; °P < .05 vs decreased weight (ANOVA followed by Fisher's LSD Multiple comparison test or chi‐squared test)
When patients were stratified according to outcome, that is, decreased/unchanged/ increased weight, changes of body weight ranged, on average, from −4.9 kg in subjects with a weight decrease, to 3.4 kg in subjects reporting a weight gain (Figure 3B and Table 5).
As reported in Table 6, no consistent physical activity was present in the majority of subjects with unchanged (86%) and increased weight (67.9%). Only the minority of subjects described a high adherence to Mediterranean diet, irrespective of outcome. However, subjects with increased weight after the lockdown reported more frequently a low adherence to Mediterranean diet.
TABLE 6.
Effect of lockdown on weight, physical activity and adherence to Mediterranean diet in the NAFLD group a , according to weight outcome
| Decreased weight | Unchanged weight | Increased weight | |
|---|---|---|---|
| N. (%) | 6 (14.6%) | 7 (17%) | 28 (68.3%)* |
| Weight (kg) | −4.9 ± 1.0 | 0 ± 0.9 ° | 3.4 ± 0.5* |
| Physical activity levels | |||
| None | 3 (50%) | 6 (85.7%) ° | 19 (67.9%)* |
| 10 min‐2.5 h/wk | 1 (16.7%) | 1 (14.2%) | 6 (21.4%) |
| >2.5 h/wk | 2 (33.3%) | 0 ° | 3 (10.7%)* |
| Mediterranean diet score | |||
| Low adherence | 1 (16.7%) | 0 (0%) ° | 8 (28.6%) ° |
| Moderate adherence | 4 (66.7%) | 6 (85.7%) ° | 18 (64.3%) |
| High adherence | 1 (16.7%) | 1 (14.3%) | 2 (7.1%) |
The group consisted of 41 overweight and obese subjects with NAFLD enrolled 1 y before the lockdown into an educational follow‐up programme aiming at healthy lifestyles, weight loss and improved NAFLD.
P < .05 vs decreased and unchanged weight.
P < .05 vs decreased weight (ANOVA followed by Fisher's LSD multiple comparison test or chi‐squared test, as appropriate).
As shown in Figure 4, fatty liver score at 3 months before lockdown decreased significantly only in the subgroup with weight loss.
FIGURE 4.
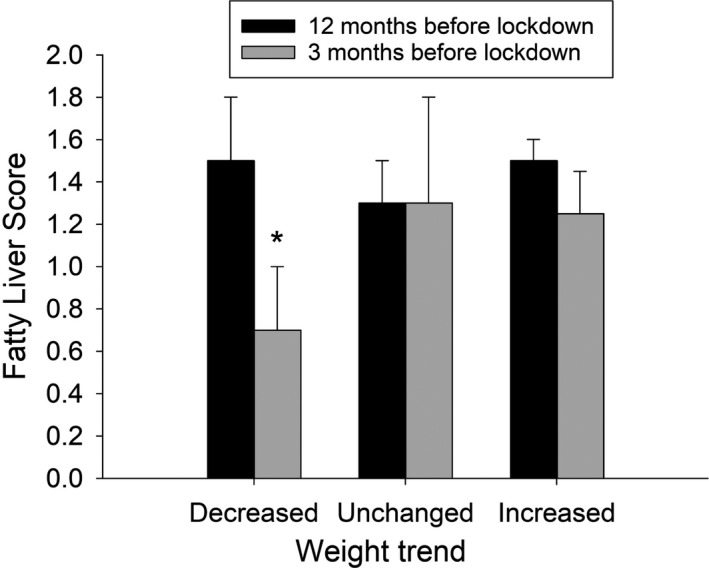
Fatty liver score measured by ultrasonography 12 and 3 mo before the lockdown in a subgroup of overweight/obese subjects enrolled, 1 y before the lockdown, in a monitoring programme for weight loss. Bars indicate means, vertical lines indicate SE. *P < .05, Student's t test for paired data
Figure 5 shows individual changes of body weight (both absolute and percentages) in subjects stratified according to outcome and the speed of weight change during the follow‐up. In subjects with weight reduction or increase, these changes appeared significantly accelerated in the last month of observation (ie during lockdown).
FIGURE 5.
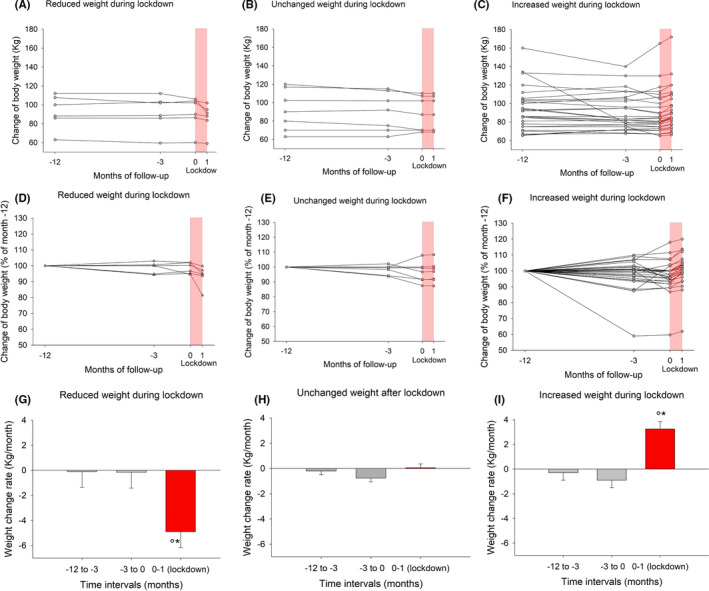
NAFLD group. Individual changes of body weight during follow‐up according to body change during lockdown (ie reduced, unchanged, increased weight). Values are presented as absolute changes (A‐C), normalized to body weight at month −12 (D‐F) and rate of weight change (G‐I). The timing of follow‐up is −12, −3, 0, and 1 mo. The lockdown period is highlighted. °P < .05 vs interval −12 to −3 mo; *P < .05 vs interval −3 to 0 mo; (ANOVA followed by Fisher's LSD multiple comparison test or chi‐squared test)
3.2.1. Lockdown effect in morbid obese patients
The lockdown period prompted us to analyse the weight trend in a further subgroup of five morbid obese patients (ie BMI > 40 kg/m2) with NAFLD, one undergoing sleeve gastrectomy bariatric surgery 2 months after start of enrolment (Figure 6A‐C). Overall, three out of five patients gained weight till lockdown. Weight loss occurred in the remaining two patients and especially in the patient receiving bariatric surgery (ie −54 kg). During lockdown, all five patients displayed weight gain with accelerated weight gain rate, ranging from 2 to 7 kg/month (Figure 6C).
FIGURE 6.
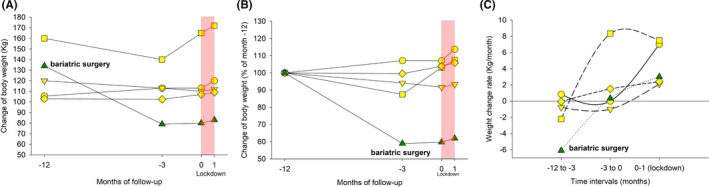
Lockdown effect in morbid obese patients. Individual weight trends in five morbid obese patients (ie BMI > 40 kg/m2) with NAFLD (one undergoing sleeve gastrectomy bariatric surgery 2 mo after start of enrolment, green triangle). The two main periods are before (−12 and −3 mo), and during lockdown (red area). A, Absolute changes of body weight during follow‐up. B, Change of body weight as per cent at entry (−12 mo). C, Weight change rate (kg/month) in the three separate periods during follow‐up. During lockdown, all five patients displayed weight gain with accelerated weight gain rate
4. DISCUSSION
The COVID‐19 pandemics prompted several governments worldwide to adopt drastic measures (ie social distancing, temporary lockdown) to decrease the deleterious consequences of diffuse viral infection. Accordingly, such preventive measures caused significant changes of lifestyle and dietary habits, with potential influence on metabolic health in the general population. In particular, a weight gain was often reported both in children 27 , 28 and in adults. 29 , 30 , 31 , 32 A recent systematic review found that the social containment measures due to the COVID‐19 lockdown generated a weight gain in 7.2%‐72.4% of subjects. 31
Modification of lifestyle by COVID‐19 lockdown caused also perceived stress, 33 worse sleep quality, 33 , 34 reduced physical activity 29 , 34 , 35 , 36 and altered dietary habits (ie variable adherence to Mediterranean diet, 29 , 33 , 37 lower meat and fish consumption, 29 high fast food consumption 29 and reduced consumption of fresh food 38 ).
The present study confirms that even a short‐term (1 month) lockdown caused fast variations in the body weight, with a weight gain in almost half of the enrolled subjects, irrespective of prior BMI. Results suggest that the weight gain observed in a large group of enrolled subjects has probably a multifactorial origin also depending on socio‐cultural and economic factors (ie education level, place of residence).
The marked alterations of food habits and lifestyle generated by the lockdown certainly played a crucial role, as also shown by the increased average number of daily meals in those reporting an increased body weight during the lockdown and by the differences recorded in physical activity and level of adherence to Mediterranean diet. These factors strongly influenced weight variations during the lockdown, as a result from an unbalance between food intake and energy consumption (ie an unhealthy lifestyle).
Previous observations linked the lockdown to stress and depression with subsequent lifestyle changes and physical inactivity. 39 These changes could be responsible, at least in part, for the weight gain observed in a subgroup of subjects enrolled in the present study. 40 An online survey conducted on Italian subjects during the lockdown (April‐May 2020) documented, in the majority of responders, anxious feeling, depression and insomnia. In these subjects, the altered mood paralleled altered feeding habits, mainly due to positive feels deriving from increased food intake. 41 Results from our study, however, does not indicate the number of sleep hours per night as linked with weight variations, since this index was similar in subjects who reported a decreased, unchanged or increased body weight.
In this study, the weight gain observed during the lockdown was associated with age, and the highest weight gain occurred in middle ages (31‐60 years), as compared to both younger and older adults. This observation is in line with a previous study in a Spanish population. 42 The low number of aged subjects can explain the weak positive correlation calculated in our series.
In the whole group of subjects, average weight changes were comparable in those with different BMI, and no correlation existed between these two variables.
Subgroups of subjects living in different countries were not representative of the whole country. However, recorded data suggested that weight gain differed in subjects of different nationalities, with greater values recorded in subjects living in Italy, as compared with those living in other countries. When subjects living in different countries were compared, a higher adherence to Mediterranean diet was recorded in subjects living in countries where the smallest weight gain was recorded. The Italian nationality, however, was the most represented in the general cohort (76.1% of enrolled subjects), and this might have generated an enrolment bias which limits the validity of this finding.
Nevertheless, the protective role of a healthy diet is supported by findings in the whole population, where weight gain was smaller in subjects reporting moderate/high adherence to Mediterranean diet than in individuals with low adherence. Of note, a recent survey in a large number of adults living in Cyprus found that, adherence to Mediterranean diet during the lockdown was only moderate, increasing only in those practicing religious fasting. 33
Performing a regular physical activity also appears as a powerful protective factor against weight gain. In fact, subjects with low levels of physical activity showed, in the present series, the highest weight gain.
Of note, the beneficial effects of physical activity are present despite the short period of observation (1 month). This is in line with previous data suggesting that both a marked weight loss and beneficial effects in terms of cardiovascular health can be achieved by short bursts of high‐intensity training. 43
The protective effect of physical activity was also evident in the subgroup of subjects included both in the present survey and, 1 year before the lockdown, in a monitoring programme aimed at obtaining weight loss through healthy lifestyles.
On average, a weight gain was also observed in these subjects. However, the majority of those who reported a weight gain had no physical activity and a low‐moderate adherence to Mediterranean diet during the lockdown.
The lockdown contributed to abort the weight loss programme in the majority of these patients, anticipating detrimental health consequences in the medium‐long term. A survey in US adults during and after the lockdown confirmed a further increase of body weight and body mass index in the post‐lockdown period, as compared with the peak lockdown period. In this series, 33% of subjects who showed a weight gain during the lockdown continued to gain weight after the lockdown, likely due to persistent inappropriate lifestyles. 32
At the meantime, data from the present study confirm the protective effect of a regular, high‐level physical activity also in terms of beneficial effects for NAFLD, as previously underscored by our group. 11 In fact, in enrolled subjects who maintained an adequate compliance to healthy lifestyles in the months before, and during the lockdown, a decreased ultrasonographic fatty liver score was evident.
Results from the present study also showed that the lockdown significantly accelerated the weight variation trend (either towards a decreased weight or a weight gain) already present in the previous months, probably amplifying previous healthy‐ or unhealthy lifestyles of the enrolled subjects. From this point of view, in particular, the limited duration of the full social restriction cannot be considered a mitigating factor able to prevent negative outcomes in terms of metabolic risk. These effects, conversely, appear to be boosted by the strict social isolation.
Evidence from the present study confirms the importance of public health intervention programmes addressing healthy habits. According to WHO, 60% of factors related to individual health and quality of life are correlated to lifestyles, 44 and unhealthy lifestyle habits can generate metabolic diseases, obesity, skeletal diseases and increase risk of disability and death. 45
These conclusions also apply to subjects with fatty liver, and results from the present study confirm previous observations documenting the role of Mediterranean diet and physical activity as additional, efficient tools in the treatment of NAFLD. 46
A collateral health risk generated by the weight gain observed during the lockdown should be related to SARS‐CoV‐2 infection. As suggested by previous evidence, obese people are more prone to COVID‐19 and related complications. 47 , 48 COVID‐19 infection and obesity should be considered as colliding points of two public health pandemics, 49 , 50 magnifying possible negative effects of both conditions on public health. It should be underlined that the coexistence of COVID‐19 infection and NAFLD represents an additional risk of liver injury. 51 On the other hand, the presence of NAFLD can affect the outcome of COVID‐19, possibly due to pathogenic inter‐relation with comorbidities, and to systemic effects of chronic inflammatory changes secondary to the expansion of metabolically active fat. 50
4.1. Limitations of the study
The present study has some limitations. First, the short observation time (1 month) might underestimate the results. In particular, the beneficial effects of a healthy diet might require a longer observation time to be fully recorded, also considering a stable compliance of subjects to dietary habits. Nevertheless, overall trends concerning weight gain rate over lockdown reveal themselves already. Second, an enrolment bias is likely, due to the limited presence of European subjects living in countries different from Italy. Results deriving from this subgroup, however, are in line with findings in the general population, mainly in terms of beneficial effects of Mediterranean diet, and regular physical activity.
Third, the number of enrolled subjects is relatively small, and no a priori power analysis was performed. This limitation was mainly due to the design of the study (observational study, based on voluntary questionnaire compilation), and to the main goal of the research protocol, which was oriented to quickly illustrate a picture of lifestyle variations in a very limited time window (ie 1 month) and during an exceptional worldwide event. The rapid evolution of the events (March 11, 2020: declaration of the pandemic; April 2020, start of the lockdown in the majority of European Countries; April 15‐May 15, data collection in the present study) did not allow us to adequately perform a preliminary power analysis and to have enough time to collect a larger number of enrolled individuals. However, we considered this rapidly imposed social containment as a unique opportunity to explore the possible negative effects of the lockdown on a trans‐national basis. Furthermore, at the moment, the number of subjects enrolled in the present study and living in different European countries (n = 494) is the largest available, when previous studies considering the lifestyle effects of the lockdown in Europe are considered.
Finally, the possibility exists that the main sampling method employed in the present study (ie web‐based questionnaire) has generated biased results, which can be only representative of people using social networks, rather than of the general public. Web‐based questionnaires, however, have been largely used to assess various health effects linked with the lockdown in European countries. 41 , 42 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 In addition, a recent study exploring the psychological impact of the pandemic and lockdown measures in subjects from seven different countries showed that a moderate use of the social networks (ie 1‐2 hours per day) was linked with less negative effects, as compared with excessive use or no use at all. Authors also underlined the role of social media as a protective factor. 60 Thus, in this context, results from the present study might have underestimated the effects possibly present in the general population and, in particular, in people who do not use social networks.
4.2. Strengths of the study
The strengths in this study include the largest multi‐national number of enrolled subjects at a European level, the possibility to compare different geographical areas using on a simple, anonymous questionnaire and the opportunity to evaluate specific changes in a subgroup of local NAFLD patients.
Previous studies exploring lifestyle and behavioural variations during the lockdown in European adults were conducted, in the majority of cases, at a national level (mainly in The Netherlands, 61 , 62 Spain, 42 , 52 Scotland, 53 Poland, 54 UK 55 Italy. 41 , 56 , 63 , 64 France, 57 Croatia, 58 Belgium 59 ), generally confirming the trend towards the unhealthy behaviours described in our study. A unique study explored an international cohort of European subjects, confirming the presence of negative effects of the lockdown on physical activity and in inducing unhealthy dietary behaviours during confinement. 65 Unfortunately, however, the European countries explored in the cited study were not specified in details, and possible trans‐national differences were not examined. Furthermore, the number of European citizens enrolled in the cited study (n = 220) was about half, as compared with the present study. Thus, our study explored, for the first time and in a relatively large number of subjects, a comprehensive panel of lifestyle aspects (ie dietary habits, physical activity, smoking habits, alcohol consumption, sleep duration), and the possibility to compare differences in 21 European countries.
Furthermore, relevant confirmation of the general results derives from the analysis performed in the subgroup of NAFLD patients, a well‐investigated group already undergoing a re‐educational programme about healthy lifestyle. Last but not least, further evidences arise from the analysis of the available subgroup of morbid obese patients, one undergoing bariatric surgery before lockdown. All patients invariably showed an accelerated weight gain during lockdown.
In conclusion, the marked social isolation paves the way to unhealthy lifestyles or acts as an amplification factor of prior metabolic risks. Even a short‐term lockdown in line with this view, contributing to exposure to risk of whole populations. With this study, we predict significant implications in terms of primary and secondary prevention, starting already during short‐term periods. In this context, we confirm that healthy lifestyles (in particular adherence to Mediterranean diet and regular physical activity) continue to play a relevant protective role in terms of weight gain and fatty liver. Thus, educational campaigns aimed at increase the awareness of beneficial effects of healthy diet and regular physical activity should invariably parallel any measure imposing social containment.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
We would like to thank Rosa De Venuto and Paola De Benedictis for their excellent technical support to this study.
Shanmugam H, Di Ciaula A, Di Palo DM, et al. Multiplying effects of COVID‐19 lockdown on metabolic risk and fatty liver. Eur J Clin Invest. 2021;51:e13597. 10.1111/eci.13597
Funding information
The present work is written in the context of the project FOIE GRAS, which has received funding from the European Union's Horizon 2020 Research and Innovation framework, under the Marie Skłodowska‐Curie Grant Agreement No. 722619. HS and EMM are recipients of Foie Gras Early Research Training Grant
Shanmugam and Di Ciaula are equal contribution.
REFERENCES
- 1. World Health Organization . Coronavirus disease 2019 (COVID 19). Situation Report ‐ 72. Geneva: World Health Organization; 2020. [Google Scholar]
- 2. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed. 2020;91(1):157‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodriguez‐Perez C, Molina‐Montes E, Verardo V, et al. Changes in dietary behaviours during the COVID‐19 outbreak confinement in the Spanish COVIDiet study. Nutrients. 2020;12(6):1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Renzo L, Gualtieri P, Pivari F, et al. Eating habits and lifestyle changes during COVID‐19 lockdown: an Italian survey. J Transl Med. 2020;18(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Ciaula A, Palmieri VO, Migliore G, Portincasa P, Group IMC . COVID‐19, internists and resilience: the north‐south Italy outbreak. Eur J Clin Invest. 2020;50(7):e13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mandel A, Veetil VP. The economic cost of Covid lockdowns: an out‐of‐equilibrium analysis. Econ Disaster Clim Chang. 2020:1‐21. 10.1007/s41885-020-00066-z [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Codagnone C, Bogliacino F, Gomez C, et al. Assessing concerns for the economic consequence of the COVID‐19 response and mental health problems associated with economic vulnerability and negative economic shock in Italy, Spain, and the United Kingdom. PLoS ONE. 2020;15(10):e0240876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moynihan AB, van Tilburg WA, Igou ER, Wisman A, Donnelly AE, Mulcaire JB. Eaten up by boredom: consuming food to escape awareness of the bored self. Front Psychol. 2015;6:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States From 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524‐530 e521; quiz e560. [DOI] [PubMed] [Google Scholar]
- 11. Molina‐Molina E, Lunardi Baccetto R, Wang DQ, de Bari O, Krawczyk M, Portincasa P. Exercising the hepatobiliary‐gut axis. The impact of physical activity performance. Eur J Clin Invest. 2018;48(8):e12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molina‐Molina E, Krawczyk M, Stachowska E, Lammert F, Portincasa P. Non‐alcoholic fatty liver disease in non‐obese individuals: prevalence, pathogenesis and treatment. Clin Res Hepatol Gastroenterol. 2019;43(6):638‐645. [DOI] [PubMed] [Google Scholar]
- 13. Zhou J, Zhou F, Wang W, et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology. 2020;71(5):1851‐1864. [DOI] [PubMed] [Google Scholar]
- 14. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274‐285. [DOI] [PubMed] [Google Scholar]
- 15. Younossi ZM. Non‐alcoholic fatty liver disease ‐ A global public health perspective. J Hepatol. 2019;70(3):531‐544. [DOI] [PubMed] [Google Scholar]
- 16. Lindenmeyer CC, McCullough AJ. The natural history of nonalcoholic fatty liver disease‐an evolving view. Clin Liver Dis. 2018;22(1):11‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rinella ME, Sanyal AJ. Management of NAFLD: a stage‐based approach. Nat Rev Gastroenterol Hepatol. 2016;13(4):196‐205. [DOI] [PubMed] [Google Scholar]
- 18. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73‐84. [DOI] [PubMed] [Google Scholar]
- 19. Di Ciaula A, Carbone F, Shanmugham H, et al. Adiponectin involved in portal flow hepatic extraction of 13C‐metacethin in obesity and non‐alcoholic fatty liver. Eur J Intern Med. 2021. 10.1016/j.ejim.2021.03.036 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20. Molina‐Molina E, Shanmugam H, Di Ciaula A, et al. ((13)C)‐Methacetin breath test provides evidence of subclinical liver dysfunction linked to fat storage but not lifestyle. JHEP Rep. 2021;3(1):100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palmentieri B, de Sio I, La Mura V, et al. The role of bright liver echo pattern on ultrasound B‐mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38(7):485‐489. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization . Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i‐xii, 1‐253. [PubMed] [Google Scholar]
- 23. National Institute of Health . Clinical Guidelines on the Identification, Evaluation, and treatment of overweight and obesity in adults‐the evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S‐209S. [PubMed] [Google Scholar]
- 24. Sofi F, Dinu M, Pagliai G, Marcucci R, Casini A. Validation of a literature‐based adherence score to Mediterranean diet: the MEDI‐LITE score. Int J Food Sci Nutr. 2017;68(6):757‐762. [DOI] [PubMed] [Google Scholar]
- 25. Dawson B, Trapp RG. Basic & Clinical Biostatistics, vol. 3rd. New York, NY: McGraw‐Hill; 2001. [Google Scholar]
- 26. Hintze J. NCSS 2020 Statistical Software (2020). Kaysville, UT: NCSS, LLC. ncss.com/software/ncss. Kaysville, Utah: Number Cruncher Statistical System (NCSS); 2020. [Google Scholar]
- 27. Vinker‐Shuster M, Grossman ES, Yeshayahu Y. Increased weight gain of children during the COVID‐19 lockdown. Isr Med Assoc J. 2021;23(4):219‐222. [PubMed] [Google Scholar]
- 28. Androutsos O, Perperidi M, Georgiou C, Chouliaras G. Lifestyle changes and determinants of children's and adolescents' body weight increase during the first COVID‐19 lockdown in Greece: the COV‐EAT study. Nutrients. 2021;13(3):930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sulejmani E, Hyseni A, Xhabiri G, Rodriguez‐Perez C. Relationship in dietary habits variations during COVID‐19 lockdown in Kosovo: the COVIDiet study. Appetite. 2021;164:105244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mulugeta W, Desalegn H, Solomon S. Impact of the COVID‐19 pandemic lockdown on weight status and factors associated with weight gain among adults in Massachusetts. Clin Obes. 2021:e12453. 10.1111/cob.12453 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan MA, Menon P, Govender R, et al. Systematic review of the effects of pandemic confinements on body weight and their determinants. Br J Nutr. 2021;1‐74. 10.1017/S0007114521000921 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhutani S, vanDellen MR, Cooper JA. Longitudinal weight gain and related risk behaviors during the COVID‐19 pandemic in adults in the US. Nutrients. 2021;13(2):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kolokotroni O, Mosquera MC, Quattrocchi A, Heraclides A, Demetriou C, Philippou E. Lifestyle habits of adults during the COVID‐19 pandemic lockdown in Cyprus: evidence from a cross‐sectional study. BMC Public Health. 2021;21(1):786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galali Y. The impact of COVID‐19 confinement on the eating habits and lifestyle changes: a cross sectional study. Food Sci Nutr. 2021;9(4):2105‐2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bogataj Jontez N, Novak K, Kenig S, Petelin A, Jenko Praznikar Z, Mohorko N. The impact of COVID‐19‐related lockdown on diet and serum markers in healthy adults. Nutrients. 2021;13(4):1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang GY, Lin XL, Fang AP, Zhu HL. Eating habits and lifestyles during the initial stage of the COVID‐19 lockdown in China: a cross‐sectional study. Nutrients. 2021;13(3):970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruggiero E, Mignogna C, Costanzo S, et al. Changes in the consumption of foods characterising the Mediterranean dietary pattern and major correlates during the COVID‐19 confinement in Italy: results from two cohort studies. Int J Food Sci Nutr. 2021;1‐13. 10.1080/09637486.2021.1895726 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38. Janssen M, Chang BPI, Hristov H, Pravst I, Profeta A, Millard J. Changes in food consumption during the COVID‐19 pandemic: analysis of consumer survey data from the first lockdown period in Denmark, Germany, and Slovenia. Front Nutr. 2021;8:635859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheikh Ismail L, Osaili TM, Mohamad MN, et al. Assessment of eating habits and lifestyle during coronavirus pandemic in the MENA region: a cross‐sectional study. Br J Nutr. 2020;1‐30. 10.1017/S0007114520004547 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marchitelli S, Mazza C, Lenzi A, Ricci E, Gnessi L, Roma P. Weight gain in a sample of patients affected by overweight/obesity with and without a psychiatric diagnosis during the Covid‐19 lockdown. Nutrients. 2020;12(11):3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Renzo L, Gualtieri P, Cinelli G, et al. Psychological aspects and eating habits during COVID‐19 home confinement: results of EHLC‐COVID‐19 Italian online survey. Nutrients. 2020;12(7):2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lopez‐Moreno M, Lopez MTI, Miguel M, Garces‐Rimon M. Physical and psychological effects related to food habits and lifestyle changes derived from Covid‐19 home confinement in the Spanish Population. Nutrients. 2020;12(11):3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Obert J, Pearlman M, Obert L, Chapin S. Popular weight loss strategies: a review of four weight loss techniques. Curr Gastroenterol Rep. 2017;19(12):61. [DOI] [PubMed] [Google Scholar]
- 44. Ziglio E, Currie C, Barnekow V. The WHO cross‐national study of health behavior in school‐aged children from 35 countries: findings from 2001–2002. J Sch Health. 2004;74(6):204‐206. [DOI] [PubMed] [Google Scholar]
- 45. Farhud DD. Impact of lifestyle on health. Iran J Public Health. 2015;44(11):1442‐1444. [PMC free article] [PubMed] [Google Scholar]
- 46. Romero‐Gomez M, Zelber‐Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829‐846. [DOI] [PubMed] [Google Scholar]
- 47. Hu X, Pan X, Zhou W, et al. Clinical epidemiological analyses of overweight/obesity and abnormal liver function contributing to prolonged hospitalization in patients infected with COVID‐19. Int J Obes (Lond). 2020;44(8):1784‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kassir R. Risk of COVID‐19 for patients with obesity. Obes Rev. 2020;21(6):e13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ryan DH, Ravussin E, Heymsfield S. COVID 19 and the patient with obesity ‐ The editors speak out. Obesity (Silver Spring). 2020;28(5):847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID‐19 and non‐alcoholic fatty liver disease: two intersecting pandemics. Eur J Clin Invest. 2020;50(10):e13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ji D, Qin E, Xu J, et al. Non‐alcoholic fatty liver diseases in patients with COVID‐19: a retrospective study. J Hepatol. 2020;73(2):451‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perez‐Rodrigo C, Gianzo Citores M, Hervas Barbara G, et al. Patterns of change in dietary habits and physical activity during lockdown in Spain due to the COVID‐19 pandemic. Nutrients. 2021;13(2):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Janssen X, Fleming L, Kirk A, et al. Changes in physical activity, sitting and sleep across the COVID‐19 National lockdown period in Scotland. Int J Environ Res Public Health. 2020;17(24):9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Blaszczyk‐Bebenek E, Jagielski P, Boleslawska I, Jagielska A, Nitsch‐Osuch A, Kawalec P. Nutrition behaviors in polish adults before and during COVID‐19 lockdown. Nutrients. 2020;12(10):3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robinson E, Boyland E, Chisholm A, et al. Obesity, eating behavior and physical activity during COVID‐19 lockdown: a study of UK adults. Appetite. 2021;156:104853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cancello R, Soranna D, Zambra G, Zambon A, Invitti C. Determinants of the lifestyle changes during COVID‐19 pandemic in the residents of Northern Italy. Int J Environ Res Public Health. 2020;17(17):6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rolland B, Haesebaert F, Zante E, Benyamina A, Haesebaert J, Franck N. Global changes and factors of increase in caloric/salty food intake, screen use, and substance use during the early COVID‐19 containment phase in the general population in France: survey study. JMIR Public Health Surveill. 2020;6(3):e19630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dogas Z, Lusic Kalcina L, Pavlinac Dodig I, et al. The effect of COVID‐19 lockdown on lifestyle and mood in Croatian general population: a cross‐sectional study. Croat Med J. 2020;61(4):309‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Constandt B, Thibaut E, De Bosscher V, Scheerder J, Ricour M, Willem A. Exercising in times of lockdown: an analysis of the impact of COVID‐19 on levels and patterns of exercise among adults in Belgium. Int J Environ Res Public Health. 2020;17(11):4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Passavanti M, Argentieri A, Barbieri DM, et al. The psychological impact of COVID‐19 and restrictive measures in the world. J Affect Disord. 2021;283:36‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mc Intyre K, Lanting P, Deelen P, et al. Lifelines COVID‐19 cohort: investigating COVID‐19 infection and its health and societal impacts in a Dutch population‐based cohort. BMJ Open. 2021;11(3):e044474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Poelman MP, Gillebaart M, Schlinkert C, et al. Eating behavior and food purchases during the COVID‐19 lockdown: a cross‐sectional study among adults in the Netherlands. Appetite. 2021;157:105002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Franco I, Bianco A, Bonfiglio C, et al. Decreased levels of physical activity: results from a cross‐sectional study in southern Italy during the COVID‐19 lockdown. J Sports Med Phys Fitness. 2021;61(2):294‐300. [DOI] [PubMed] [Google Scholar]
- 64. Cicero AFG, Fogacci F, Giovannini M, et al. COVID‐19‐related quarantine effect on dietary habits in a northern Italian rural population: data from the Brisighella heart study. Nutrients. 2021;13(2):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ammar A, Brach M, Trabelsi K, et al. Effects of COVID‐19 home confinement on eating behaviour and physical activity: results of the ECLB‐COVID19 international online survey. Nutrients. 2020;12(6):1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta‐analysis and a proposal for a literature‐based adherence score. Public Health Nutr. 2014;17(12):2769‐2782. [DOI] [PMC free article] [PubMed] [Google Scholar]


