Abstract
Background
Balancing the use of antibacterial therapy against selection for resistance in this pandemic era has introduced both questions and guidelines. In this project, we explored how prescription of empirical antibacterial therapy differs between those with and without SARS-CoV-2 infection.
Methods
Multivariable logistic regression was used to determine whether COVID-19 status and other factors play a role in the prescription of antibacterial therapy in an inpatient setting at a large referral academic medical centre. Further analysis was conducted to determine whether these factors differ between those testing positive and negative for SARS-CoV-2.
Results
Of 405 patients in the cohort, 175 received antibacterial therapy and 296 tested positive for SARS-CoV-2. A positive SARS-CoV-2 test carried an OR of 0.3 (95% CI: 0.19, 0.49) for receiving antibacterial treatment in the first 48 h after admission (P < 0.0001) adjusting for age and procalcitonin results. Patients were 1% and 3% less likely to receive antibacterials for every year increase in age in the overall group and among those testing negative for SARS-CoV-2, respectively. Younger age was found to impact use of antibacterial therapy in both the overall analysis as well as the SARS-CoV-2 negative subgroup (P = 0.03 and P = 0.01). High procalcitonin values were found to be associated with increased antibacterial therapy use in both the overall and stratified analyses.
Conclusions
Antibacterial therapy prescription differs by COVID-19 disease status, and procalcitonin results are most highly associated with antibacterial use across strata.
Introduction
As the SARS-CoV-2 pandemic rages, questions on the role of antibacterial therapy among COVID-19 patients persist. SARS-CoV-2 patients who experience bacterial coinfection suffer higher severity of disease and mortality, but rampant use of antimicrobials must be balanced against the selection for antimicrobial resistance.1 Clinicians have called for clear guidance regarding antibacterial drug prescribing in those infected with SARS-CoV-2.1–3 While WHO, IDSA and others have published guidelines, practice patterns are still evolving.4,5
In an exploratory analysis of our prospective observational cohort, we observed fewer courses of antibacterial therapy in patients who entered isolation care with COVID-19 rather than those suspect cases that ultimately were SARS-CoV-2 negative.6 In this project, we sought to understand how COVID-19 disease status affected clinical decision-making on the use of empirical antibacterial therapy for newly hospitalized patients being assessed for COVID-19 and sepsis. We did this to test our exploratory finding in the observational cohort against the broader hospital patient census and, depending upon the results, to assist either revisions in isolation care guidance or prospective study design, as appropriate.
In undertaking this work, we drew upon electronic health record variables that could be reliably obtained, are common in assessments for the use of antibacterial therapy and might lend themselves to use in corrective education or guidelines refinement. Procalcitonin, in particular, was of interest.7–9 In our cohort, while numbers were low, results in COVID-19 patients were variable regardless of bacterial infection status. This provided an opportunity to verify this finding and address pre-conceived notions of the biomarker’s performance, while also employing it to explore manifestations of prescriber attitudes that may require intervention.
Materials and methods
Study population
The Clinical Characterization Protocol for Severe Emerging Infections (CCPSEI) at the University of Nebraska Medical Center (UNMC) and Nebraska Medicine (NM) was approved by the single institutional review board, 146-20-FB. CCPSEI participants completed informed consent. Deidentified data from the larger dataset of hospitalizations did not require informed consent. We identified patients admitted to Nebraska Medicine between April and October of 2020 who had a nucleic acid test result for SARS-CoV-2 (conventional real-time PCR or BioFire FilmArray®) within 72 h of admission. For the primary analysis, the dataset was further restricted to patients who had procalcitonin values within 5 days of admission as a surrogate for concern for sepsis to eliminate pre-procedural and other SARS-CoV-2 screening events, as well as to allow for assessment of this biomarker.
Exposures
The primary variable of interest was COVID-19 disease status defined as positive or negative SARS-CoV-2 nucleic acid test result within 72 h of admission. Gender (male or female), procalcitonin test result, presence of bacterial isolate from any specimen collected within 5 days and race were also included. Procalcitonin test results were defined as positive for values greater than or equal to 0.50 ng/mL and negative for values less than 0.50 ng/mL per UNMC guidance.7 Presence of culture-confirmed bacterial isolate was defined as a positive culture result with sufficient colony count (BAL or urine with a single isolate greater than 10 000, sputum greater than 100 000) or qualitative growth in respiratory samples as moderate or higher; a positive PCR result with equivalent copies detected (BioFire® BCID); or any detection from a sterile site. Clinical adjudication was not conducted as this was an expanded de-identified electronic health record dataset. However, positive bacterial test results were excluded if recovered normal flora or if isolates were classified by the microbiology lab as contaminants. Due to incompleteness in race and ethnicity data, race was categorized as white, black, Asian or other (American Indian or Alaska Native, Hispanic, Native Hawaiian or other Pacific Islander, and unknown). Age and WBC count lab values were captured as continuous variables.
Outcome
The outcome was whether antibacterial therapy was administered within 48 h of admission in order to focus on empirical therapy for the presenting clinical syndrome. Antibacterial agents were restricted to those administered orally, intravenously and by inhalation. Prophylactic antibacterial therapy for procedures or underlying comorbidity were excluded.
Statistical analysis
Data were analysed using SAS® version 9.4. Descriptive epidemiology, bivariate analysis using t-tests for continuous variables and χ2 tests for categorical variables were conducted on each exposure variable to determine unadjusted association with use of antibacterial agents. Variables meeting a P value threshold of 0.20 were considered for inclusion in multivariable logistic regression for the primary analysis. Backward selection was then utilized to eliminate statistically insignificant variables at an alpha of 0.05. An assessment of precision was conducted at each step of the selection process.
Stratified analyses were then conducted to explore how factors associated with antibacterial prescription differ between patients with and without COVID-19. To accomplish this, the same method as outlined for the primary analysis was used on SARS-CoV-2 positive and SARS-CoV-2 negative patient data.
A secondary analysis was conducted to determine what factors were associated with having a procalcitonin value as a means of identifying potential bias introduced by restricting the dataset by this variable. To accomplish this, a new dichotomous variable was created indicating whether a value for procalcitonin existed, this variable was used as the outcome with all other variables acting as exposures. Mirroring the primary analysis, variables meeting a P value threshold of 0.20 were considered for inclusion in multivariable logistic regression model, and backward selection was used to eliminate variables at an alpha of 0.05.
Results
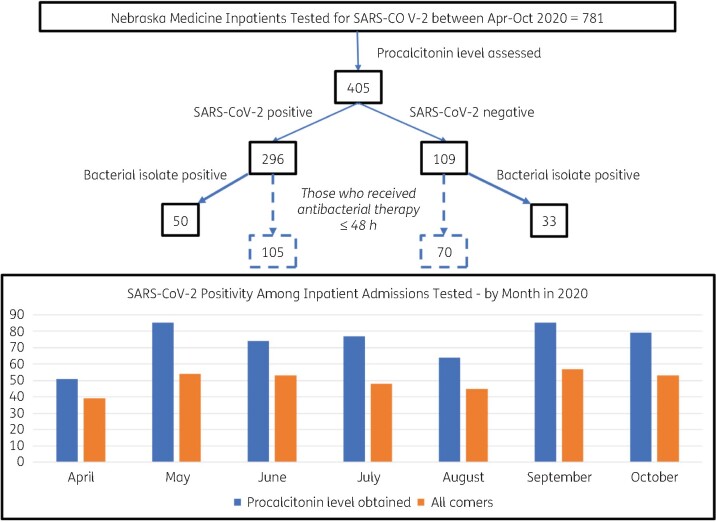
Month of admission ranged from April to October of 2020 with an even distribution across months. Each month, admissions tested for SARS-CoV-2 varied between 1 in 10 and 1 in 5 of all admissions. Figure 1 contains information on how the population was restricted, as well as percentage of SARS-CoV-2 test positivity among these patients both restricted and unrestricted by the presence of a procalcitonin biomarker. Demographics and distribution of other factors among those given and not given antibacterial therapy are shown in Table 1. Of 405 patients tested for both procalcitonin and SARS-CoV-2, 175 (43%) received antibacterial therapy in the first 48 h after admission, and 296 (73%) tested positive for SARS-CoV-2. Specific race was not known for more than half of persons categorized as other. Status as Hispanic ethnicity was poorly resolvable with omission and overlap issues. Fifty SARS-CoV-2 positive individuals were found to have a positive bacterial isolate present, five of which had more than one sample type positive. These were comprised of 21 blood (10 of which were CoNS or non-specified Staphylococcus; not flagged as contaminant by the laboratory), 15 urine, 15 respiratory and 4 other samples. Among the 33 SARS-CoV-2 negative individuals with a bacterial isolate identified, 8 had more than one sample type positive. These were comprised of 23 blood (5 of which were CoNS or non-specified Staphylococcus; not flagged as contaminant by the laboratory), 9 urine, 2 respiratory and 8 other samples.
Figure 1.
Down-selection of dataset and patient positivity by month.
Table 1.
Descriptive epidemiology and bivariate analysis
| Descriptive epidemiology |
Bivariate analysisa |
|||
|---|---|---|---|---|
| overall (405) | antibacterials (175) | no antibacterials (230) | association with antibacterial therapy, P value | |
| Age, years, mean (SD) | 60.1 (16.8) | 58.2 (17.5) | 61.6 (16.1) | 0.042 |
| WBC count, 1000 cells/µL, mean (SD) | 11.5 (7.2) | 13.2 (9.2) | 10.2 (4.7) | <0.0001 |
| Procalcitonin, positiveb, n (%) | 159 (39) | 96 (55) | 63 (27) | <0.0001 |
| Gender, male, n (%) | 223 (55) | 102 (58) | 121 (53) | 0.255 |
| SARS-CoV-2 positive, n (%) | 296 (73) | 105 (60) | 191 (83) | <0.0001 |
| Bacterial isolate, n (%) | 83 (20) | 47 (27) | 36 (16) | 0.006 |
| Race, n (%) | ||||
| white | 236 (58) | 108 (62) | 128 (56) | 0.599 |
| black | 56 (14) | 21 (12) | 35 (15) | |
| Asian | 9 (2) | 3 (2) | 6 (3) | |
| other | 104 (26) | 43 (25) | 61 (27) | |
Values in bold denote significance at an alpha of 0.05.
Bivariate association determined for continuous variables with t-tests and categorical variables with χ2 tests.
Positive result indicates a procalcitonin level >0.50 ng/mL.
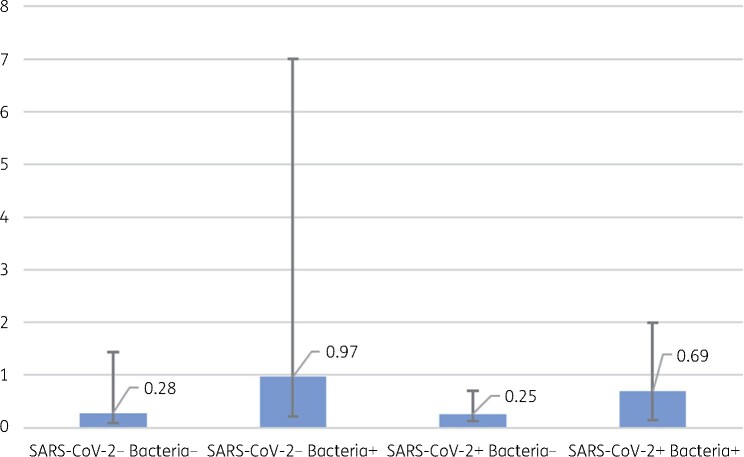
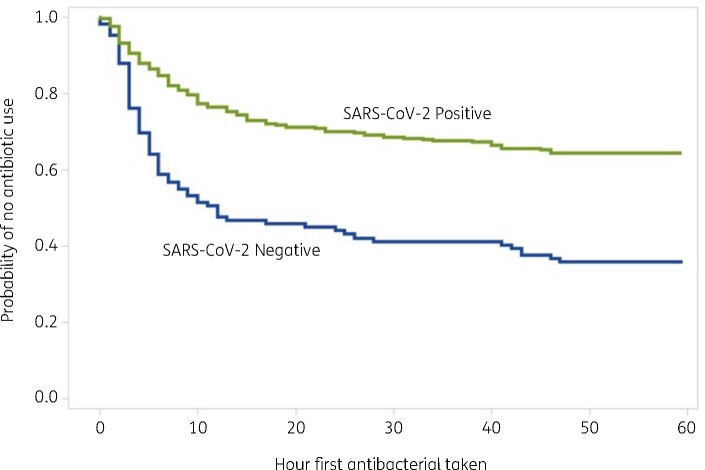
Median procalcitonin level among SARS-CoV-2 positive patients was 0.26 (IQR = 0.85–0.13) compared with 0.38 (IQR = 2.49–0.11) among negative patients, this difference did not reach statistical significance in a two-sided Mann–Whitney test for non-parametric distribution (P = 0.0768). This association showing differences in procalcitonin values stratified by COVID-19 disease status and presence of bacterial isolate is shown in Figure 2. Patients testing positive for SARS-CoV-2 who were prescribed antibacterial therapy received their first dose on average 12 h after admission, compared with 10 h after admission in those testing negative. In a Kaplan–Meier survival analysis, there was no significant difference in time to first dose of antibacterial therapy between those testing positive and negative for SARS-CoV-2 (P = 0.285). The survival curve for this association is shown in Figure 3.
Figure 2.
Procalcitonin results by SARS-CoV-2 and bacterial isolate presence. The bars represent the IQR of procalcitonin for each category. These differences were tested using a two-sided Mann–Whitney test for non-parametric distribution. The median of each range is shown above the bars.
Figure 3.
Time to first dose of antibacterial therapy by SARS-CoV-2 test result.
Table 1 includes results from bivariate analysis comparing use of antibacterial therapy among all patients to various biomarkers. Younger age (P = 0.042), increased WBC count (P < 0.0001), positive procalcitonin result (P < 0.0001), negative SARS-CoV-2 test (P < 0.0001) and presence of culture-confirmed bacterial isolate (P = 0.006) were correlated with increased use of antibacterial therapy. Multivariable logistic regression results are also shown in Table 2. WBC count and presence of bacterial isolate were removed from the regression for a lack of adjusted statistical significance. Patients who tested positive for SARS-CoV-2 were 0.30 times as likely to be given antibacterial therapy compared with their SARS-CoV-2-negative counterparts after adjusting age and procalcitonin test result (P < 0.0001).
Table 2.
Results from final multivariable logistic regression analyses
| Primary analysis |
Secondary analysis: SARS-CoV-2 positive |
Secondary analysis: SARS-CoV-2 negative |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| SARS-CoV-2, positive | 0.30 (0.19, 0.49) | <0.0001 | ||||
| Procalcitonin, positivea | 3.20 (2.08, 4.94) | <0.0001 | 3.17 (1.93, 5.23) | <0.0001 | 2.65 (1.11, 6.36) | 0.020 |
| Age, years | 0.99 (0.97, 1.00) | 0.039 | — | 0.97 (0.94, 0.99) | 0.015 | |
ORs for use of antibacterial presented.
Dashes present when variable was removed during backward selection of the statistical model as described in methods, and so not present for analysis.
Positive result indicates a procalcitonin level >0.50 ng/mL.
Next, an analysis was conducted to explore how factors affecting use of antibacterial therapy differ between patients with and without COVID-19; the resulting ORs can be found in Table 2. In these analyses of SARS-CoV-2 positive and negative groups that included backward selection of factors and check for collinearity between variables, several differences were observed. COVID-19 patients were 3.2 (95% CI: 2.1, 4.9) times as likely to receive antibacterial therapy when they had a positive procalcitonin result (P < 0.0001). In contrast, patients not confirmed to have COVID-19 were 2.7 (95% CI: 1.1, 6.4) times as likely to receive antibacterial therapy when they had a positive procalcitonin (P = 0.029). In addition, patients who tested negative for SARS-CoV-2 were 3% (95% CI: 1% to 6%) less likely to receive antibacterial therapy for every 1 year increase in age (P = 0.005).
In order to assess whether differences in use of procalcitonin among patient groups may have influenced our ability to best characterize its association with antibacterial therapy, a bivariate analysis was then conducted to determine what factors influence the presence or absence of a procalcitonin value. To accomplish this, the dataset prior to restriction based on presence of procalcitonin value was utilized. Of 781 patients included in this dataset, 405 (52%) had procalcitonin values ordered. Positive SARS-CoV-2 test (P < 0.0001), male gender (P = 0.037), presence of bacterial isolate (P < 0.0001), race (P = 0.0001), antibacterial therapy prescribed (P = 0.001) and older age (P < 0.0001) were all associated with presence of a procalcitonin test result. WBC count was the only variable that failed to achieve statistical significance in bivariate analysis. Gender, bacterial isolate and race were all removed from the multivariable logistic regression model for failing to meet statistical significance, while COVID-19 status, antibacterial therapy prescribed and age were all retained. (Table 3) Patients who tested positive for SARS-CoV-2 were 10.6 times as likely to have a procalcitonin value ordered in the first 5 days after admission when adjusting for antibacterial therapy prescription and age (P < 0.0001). Those prescribed antibacterial therapy in the first 3 days after admission were 2.8 times as likely to have a procalcitonin value drawn (P < 0.0001). Patients were 2% more likely to have a procalcitonin value for every 1 year increase in age (P < 0.0001).
Table 3.
Factors associated with presence of procalcitonin biomarker
| OR (95%CI) | P value | |
|---|---|---|
| SARS-CoV-2 positive | 10.58 (7.41, 15.1) | <0.0001 |
| Antibacterials, yes | 2.79 (1.94, 4.01) | <0.0001 |
| Age, years | 1.02 (1.01, 1.03) | <0.0001 |
Values in bold denote significance at an alpha of 0.05.
Discussion
After observing differences in antibacterial therapy practices between suspected and confirmed patients within our COVID-19 cohort, we sought to better understand how COVID-19 status affects use of inpatient empirical antibacterial therapy.8 In our centre’s patient experience, COVID-19 negative status, younger age and a positive procalcitonin test result were each associated with administration of antibacterial therapy in adjusted analyses. In our restricted line list of cases meant to represent admitted COVID-19 tested patients who were suspected of having sepsis, only 35% of those with a positive test for SARS-CoV-2 were provided antibacterial therapy in the initial phase of their hospitalization, lower than found in other hospital settings.9 Patients testing positive for SARS-CoV-2 with the presence of the procalcitonin biomarker experienced a bacterial infection rate of about 16%, which is consistent with other published works.1,10,11 The procalcitonin association is consistent with recent studies in the United Kingdom and Australia that show strong associations between procalcitonin value and antibacterial therapy in the care of COVID-19 patients.12,13
Elevated procalcitonin test results were the factor most consistently associated with use of antibacterial medications, more strongly among those with COVID-19. While procalcitonin levels were higher among those without COVID-19, the IQR extended above the test positive threshold in COVID-19 patients with and without the presence of a positive bacterial culture and so presumptions about normal procalcitonin levels in COVID-19 patients without superimposed bacterial infection should be applied with caution and require additional characterization. Younger age was associated with higher rates of antibacterial prescription in those testing negative for SARS-CoV-2, but not those testing positive. This finding may reflect clinician presumptions about who should and should not appear sick, and regardless warrants further analysis. The distribution in age between these groups was similar.
Patients testing positive for SARS-CoV-2 were more likely than those who ultimately tested negative to have a procalcitonin value assessed. In our centre, nearly all the patients represented in our dataset passed through a clinical assessment in isolation care until their SARS-CoV-2 test results were known. This exposed both those with and without COVID-19 but presenting with suspected sepsis to similar practice norms. While our dataset did not facilitate comparisons of severity and details of the presenting clinical syndrome, potential reasons for this finding include that COVID-19 patients may have presented with syndromes compatible with non-focal sepsis, or, paradoxically, that higher clinical suspicion for COVID-19 led to increased vigilance for concomitant bacterial infection that might be missed because of the focus on COVID-19, in particular community-acquired pneumonia. Additionally, procalcitonin use in the evaluation of pneumonia is encouraged in our centre. Those with higher age were also more likely to have procalcitonin levels assessed, suggesting increased concern for bacterial infection and/or its consequences in older patients. This may have been coupled with less expectation for moderate to severe COVID-19 in younger patients, precipitating more empirical antibacterial therapy use. Unfortunately, more severe COVID-19 disease in younger patients is increasingly recognized.14 It is possible that part of the reason why younger age was associated with more antibacterial therapy use is the additional information this increased procalcitonin use provided in the management of older patients. This finding contrasts with a recent meta-analysis that found more antibacterial therapy use in older patients.15 Taken together, systematic employment of a biomarker like procalcitonin may assist in increasing scrutiny on antibacterial therapy use in younger patients with COVID-19, and should be considered.
We assessed the application of empirical antibacterial therapy within 48 h of admission. While our institution’s turnaround time for SARS-CoV-2 test results is counted in hours, our dataset was not conducive to reliable fine time course analysis on when those results were made available against timing of antibacterial orders. Additionally, in our care setting, a completed assessment for COVID-19 did not reliably hinge on a single test result, or even serial results. Cases with a high pre-test probability either from the nature of the clinical syndrome (severe acute respiratory disease not otherwise explained) or a strong epidemiological link required two tests to be negative, and in other cases would be presumed positive regardless of test result. While this was a minority of cases, this reality calls for prospective research to fully capture qualitative aspects of clinical decision-making on antibacterial therapy. Other limitations of this work include the introduction of selection and misclassification biases by employing procalcitonin value as a surrogate when testing for SARS-CoV-2 for clinician concern of bacterial infection, as well as any approach to managing CoNS blood cultures without clinical adjudication, though the same challenges may occur with other pathogens.16 We chose to exclude fungal infections and empirical antifungal therapy, though each of those instances were few. We did not capture prescribing provider attitudes and reasoning associated with use, nor other metrics of antibiotic use. Our centre has usual, proactive mechanisms of antimicrobial stewardship, though whether these pandemic isolation care findings can be generalized to other settings is a matter for further study.17 Our dataset lacked sufficient power to allow time course analysis and so potential learning by or changes in COVID-19 awareness in providers over the period of observation—variability in antibacterial therapy use in COVID-19 over time has been described.15 Races in the ‘other’ category lacked sufficient numbers to explore each of their associations, and other vulnerability measures were not included. The data format did not allow an evaluation of Hispanic ethnicity, an important demographic variable in our patient population. Those with Hispanic ethnicity were represented to or above population levels among admissions for suspected and confirmed COVID-19 disease (separate electronic health record quality review, data not shown), and so were represented in our dataset. The expanded dataset included all hospitalized patients without discriminating disease severity.
A substantial aspect of COVID-19 management for both suspected and confirmed cases is normalized practice in isolation care. COVID-19 teams increase case review and dialogue between hospitalist medicine and critical care, incorporating practice norms regarding antibacterial therapy use as well as targeted advice from a consulting infectious diseases physician. These structural features may have contributed to conservative use of antibacterial therapy in our context and limit applicability of these findings when isolation care is not employed, though in non-isolation care settings conventional antimicrobial stewardship practices remain available. This work focused on antibacterial use practice and associated factors, not appropriateness. Nonetheless, as groups continue to develop and assess best recommendations for empirical antibacterial therapy in COVID-19 case management, uptake of antimicrobial stewardship practices as well as the impact of biomarker and demographic factors on decision-making should be further evaluated.
Conclusions
Among patients admitted to the hospital with suspected or confirmed COVID-19, those with SARS-CoV-2 infection received less antibacterial therapy than those who tested negative for SARS-CoV-2. Procalcitonin results may have impacted this use, particularly in those who ultimately tested negative. Younger age was associated with higher rates of antibacterial therapy in SARS-CoV-2 negative patients. Striking the balance between over- and under-use of antibacterial therapy in COVID-19 management requires clinical decision guidance that accounts for context, incorporating setting, demographic factors and employment of validated biomarkers.
Acknowledgements
The authors are grateful to Nebraska Medicine’s patients and healthcare workers, and the broad UNMC| NM CCPSEI investigator group.
Funding
This project received intramural funding support, including a Rapid Response Grant from the UNMC College of Medicine.
Transparency declarations
None to declare.
Disclaimer
These views are the authors and do not necessarily reflect the State of Nebraska or any of its agencies.
References
- 1. Rawson TM, Moore LS, Zhu N. et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020; 71: 2459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fattorini L, Creti R, Palma C. et al. Bacterial coinfections in COVID-19: an underestimated adversary. Ann Ist Super Sanita 2020; 56: 359–64. [DOI] [PubMed] [Google Scholar]
- 3. Getahun H, Smith I, Trivedi K. et al. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull World Health Organ 2020; 98: 442–442A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1.
- 5. Bhimraj A, Morgan RL, Shumaker AH. et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis 2020; doi:10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brett-Major DM, Schnaubelt ER, Creager HM. et al. Advanced preparation makes research in emergencies and isolation care possible: the case of novel coronavirus disease (COVID-19). Am J Trop Med Hyg 2020; 102: 926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Department of Internal Medicine, University of Nebraska Medical Center. Procalcitonin (PCT) Guidance. https://www.unmc.edu/intmed/divisions/id/asp/procalcitonin-pct-guidance/index.html.
- 8. Broadhurst MJ, Brett-Major D, Clinical Characterization Protocol for Severe Emerging Infections (CCPSEI). https://www.unmc.edu/publichealth/departments/epidemiology/CCPSEI.html.
- 9. Vaughn VM, Gandhi T, Petty LA. et al. Empiric antibacterial therapy and community-onset bacterial co-infection in patients hospitalized with COVID-19: a multi-hospital cohort study. Clin Infect Dis 2020; doi:10.1093/cid/ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langford BJ, So M, Raybardhan S. et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26: 1622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Townsend L, Hughes G, Kerr C. et al. Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC Antimicrob Resist 2020; 2: dlaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drewett GP, Smibert OC, Holmes NE. et al. The use of procalcitonin as an antimicrobial stewardship tool and a predictor of disease severity in COVID-19. medRxiv 2021; doi:10.1101/2021.01.14.21249853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams EJ, Mair L, de Silva TI. et al. Evaluation of procalcitonin as a contribution to antimicrobial stewardship in SARS-CoV-2 infection: a retrospective cohort study. J Hosp Infect 2021; 110: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan H, More Young People are Getting Hospitalized as a ‘Stickier,’ More Infectious Coronavirus Strain Becomes Dominant. 2021. https://www.cnn.com/2021/04/12/health/b117-covid-variant-young-patients/index.html.
- 15. Langford BJ, So M, Raybardhan S. et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect 2021; 27: 520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liaquat S, Baccaglini L, Haynatzki G. et al. Clinical consequences of contaminated blood cultures in adult hospitalized patients at an institution utilizing a rapid blood-culture identification system. Infect Control Hosp Epidemiol 2020; doi:10.1017/ice.2020.1337. [DOI] [PubMed] [Google Scholar]
- 17. Van Schooneveld TC, Rupp ME.. Antimicrobial stewardship strategies: preauthorization or postprescription audit and feedback? Infect Control Hosp Epidemiol 2014; 35: 1100–2. [DOI] [PubMed] [Google Scholar]