Abstract
Background
Antibiotic use requires regular monitoring to prevent emergence of antibiotic resistance.
Objectives
To assess antibiotic prescribing patterns at health care facilities (HCF) in Ilala district, Tanzania.
Methods
A 1 year retrospective study was conducted in four HCFs using WHO/International Network of Rational Use of Drugs (INRUD) core prescribing indicators. Factors associated with antibiotic prescription were analysed using logistic regression model.
Results
A total of 604 prescriptions were reviewed. Patients had median age (IQR) of 15 (4–31) years with majority having upper respiratory tract infection 33.3% (n = 201), urinary tract infection 31.1% (n = 188) or diarrhoea 21.2% (n = 128). Out of 624 prescribed antibiotics, amoxicillin was the most common (22.7%), followed by ciprofloxacin (13.6%) and metronidazole (11.6%). The studied HCFs had an average of 1.99 medicines prescribed per consultation (reference: 1.6–1.8). Of 1203 medicines prescribed, 51.9% (n = 624) were antibiotics (reference: 20.0%–26.8%). Additionally, 97.6% (n = 609) of the antibiotics appeared on the national essential medicines list, whereby 84.4% (n = 510) were prescribed by generic names (reference: 100%). Patients with peptic ulcers had a 4.4-fold higher chance of receiving antibiotics [adjusted odds ratio (aOR) = 4.4, 95% CI = 1.918–10.13, P = 0.0001] while patients with diarrhoea had a 2.6-fold higher chance of receiving at least one antibiotic (aOR = 2.6, 95% CI = 1.206–5.491, P = 0.015).
Conclusions
We found inappropriate use of antibiotics in the studied primary HCFs. Antibiotic stewardship programmes should be extended to primary HCFs found in Ilala district.
Introduction
Antibiotic use remains one of the most cost‐effective health interventions in the fight against infectious diseases caused by bacteria.1 However, inappropriate antibiotics use may result in the emergence of resistant bacteria. This in turn threatens the achievements made in the past decades in the prevention and control of infectious diseases.2 A resistant bacterium requires expensive antibiotics and long hospitalization period, which has both health and financial consequences in human medicine.3
To prevent the emergence of antibiotic resistance and its effects on human health, WHO recommends routine monitoring of antibiotic use.4 In 1993, the WHO in collaboration with the International Network of Rational Use of Drugs (INRUD) formulated a list of selected indictors to investigate drug use in health facilities.5 The WHO/INRUD developed core prescribing indicators, such as an average number of medicines prescribed per consultation (optimal value 1.6–1.8), the percentage of drugs prescribed by generic name (optimal value 100%), the percentage of encounters where an antibiotic was prescribed (optimal value 20.0%–26.8%), the percentage of encounters where injections were prescribed (optimal value 20.0%–26.8%) and medicines prescribed from the Essential Drugs List (optimal value 100%).4,5
Despite the existence of well-established standards for guiding the prescription practice at health care facilities (HCF), several studies have indicated substantial overuse of common antibiotics across developing countries, particularly in primary healthcare centres.6 To this end, Tanzania as an implementing partner has a National Essential Medicines List (NEML) and treatment guidelines that guide the use of antibiotics at the HCF level.7 However, the current information on antibiotic prescribing practice in Tanzania is limited. Therefore, we aimed to study antibiotic prescribing indicators and utilization patterns among outpatients attending care at primary health centres in Ilala district.
Methods
Study design, period and settings
This was a 1 year retrospective cross-sectional study, data from September 2018 to September 2019 was used in assessing antibiotic prescribing practice and utilization patterns in health centres at four primary health care centres in Ilala District, Tanzania. The study was conducted from October 2019 to January 2020. Ilala district (found in the Dar es Salaam region) has a total population of 1 220 611 with a very large population change of about 92.2% from 2002 to 2012.8 The district has about 20 health centres at the time of conducting this study; three public and one private were selected based on the availability of an electronic system known as the Government of Tanzania-health online management information system (GOT-HOMIS). Additionally, we included health centres that have installed and using GOT-HOMIS for more than 1 year.
Study population
This study was conducted in among outpatients who represents about 90% of all antibiotic usage.9 Data about study participants were collected from the patients’ electronic files through GOT-HOMIS. Information about age, sex, infectious conditions (suspected or confirmed infection), investigation, drugs prescribed and payment plan were collected. WHO recommends that there should be at least 600 encounters included in a cross-sectional survey.10 We included four primary health care centres, with about 150 encounters per facility. Four health care facilities (HCFs) were selected randomly from the Ilala district list of primary health care centres in Tanzania, but the presence of GOT-HOMIS determined their final inclusion. The sampling ensured the inclusion of both public and private primary health care centres. The number of encounters per HCF was systematically selected to have at least 150 samples from each facility. The sampling interval ‘n = 4’ was determined as the proportion of 600 encounters over 150 samples. Then patient’s file was sampled after every ‘nth’ interval.
Data analysis
Data were collected using an Open Data Kit (ODK software, USA) by adapting the WHO data collection guideline5 as previously study done by Mashalla et al.10 Data were exported from the ODK server to a Microsoft Excel sheet (Redmond, WA) then exported to a statistical package for social science (SPSS version 25, Chicago Inc., USA) for analysis. Descriptive statistics such as frequencies, percentages, averages (SD) and median (IQR) were summarized using frequency distribution tables. The predictors for laboratory investigation and prescription of antibiotics were determined using binary logistic regression model. Factors with P value <0.2 in univariate qualified for multivariate analysis. A P value <0.05 was considered statistically significant with 95% CI. Relationship between antibiotics classes and the disease conditions were presented using 2-D dot-plot graph. In order to assess the prescribing practices using WHO/INRUD prescribing indicators (average number of medicines prescribed per consultation, the percentage of drugs prescribed by generic name, the percentage of encounters where an antibiotic was prescribed, the percentage of encounters where injections were prescribed and medicines prescribed from the Essential Drugs List), the optimal values/reference standards were adopted from a previous study.11
Ethics
Ethics clearance was obtained from Muhimbili University of Health and Allied Sciences Ethical Committee (Reference number: DA.25/111/01/). Additionally, research permission was sought from the Ilala District Medical Officer, and upon entry to the respective ward and health centre, at each study site permission was sought from the Medical Officer in charge of the respective health facility. Privacy and confidentiality were observed, the identity of the participants was not recorded from the dataset used to store the information of health records at the healthcare facility.
Results
Characteristics of participants and selected clinical parameters
A total of 604 prescriptions were reviewed with most of the patients being female (54.1%; n = 327). Patients had a median age (IQR) of 15 (4–31) years with most of the patients having attended the public health centres 75% (n = 435). One-third of patients were suspected to have upper respiratory tract infection (URTI) 33.3% (n = 201) (Table 1).
Table 1.
Participants sociodemographic and clinical characteristics
| Characteristics | Frequency (n) | Percentage (%) |
|---|---|---|
| Sex | ||
| Female | 327 | 54.1 |
| Male | 277 | 45.9 |
| Age group, years, [median (IQR): 15 (4–31)] | ||
| 5 | 198 | 32.8 |
| 5–14 | 98 | 16.2 |
| 15–24 | 84 | 13.9 |
| 25–44 | 144 | 23.8 |
| 45–65 | 59 | 9.8 |
| 65 | 21 | 3.5 |
| Health facility category | ||
| Public | 453 | 75 |
| Private | 151 | 25 |
| Payment method | ||
| Insurance | 194 | 32.1 |
| User fee | 410 | 67.9 |
| Laboratory investigation done | ||
| Yes | 321 | 53.1 |
| No | 283 | 46.9 |
| Number of laboratory investigations | ||
| None | 283 | 46.9 |
| One | 86 | 14.2 |
| Two | 136 | 22.5 |
| Three | 99 | 16.4 |
| Common disease conditions | ||
| URTI | 201 | 33.3 |
| UTI | 188 | 31.1 |
| Diarrhoea | 128 | 21.2 |
| Skin infection | 72 | 11.9 |
| Peptic ulcers | 15 | 2.5 |
| Number of antibiotics per prescription | ||
| None | 80 | 13.2 |
| 1 | 434 | 71.9 |
| 2 | 90 | 14.2 |
URTI, upper respiratory tract infection; UTI, urinary tract infection.
Selected WHO/INRUD prescribing indicators
A total of 1203 drugs were prescribed from the 604 analysed prescriptions. The studied health centres in Ilala district had an average of 1.99 (SD = 0.7) medicines prescribed per consultation and an average of 84% (n = 510) of the antibiotics were prescribed by generic name. Additionally, 51.9% (n = 624) of all outpatient encounters was prescribed antibiotics during the reviewed consultations. In these primary health facilities, on average 97.6% (n = 609) of the antibiotics appeared on the national essential drugs list while 3.2% (n = 38) of the encounters were prescribed injections (Table 2).
Table 2.
The selected core WHO/INRUD prescribing indicators
| Parameter | Frequency | Percentage (%) | Reference standards11 |
|---|---|---|---|
| Average number of drugs per encounter | 1.99 (SD: 0.7) | 1.6–1.8 | |
| Encounters prescribed antibiotics | 624 | 51.9 | 20.0%–26.8% |
| Encounters prescribed injections | 38 | 3.2 | 13.4%–24.1% |
| Antibiotics prescribed are generics | 510 | 84.4 | 100% |
| Antibiotics prescribed in the essential medicine lista | 609 | 97.6 | 100% |
| Total number of prescriptions analysed | 604 | ||
| Total number of drugs prescribed | 1203 | ||
National Essential Medicines List.7
Commonly prescribed antibiotics
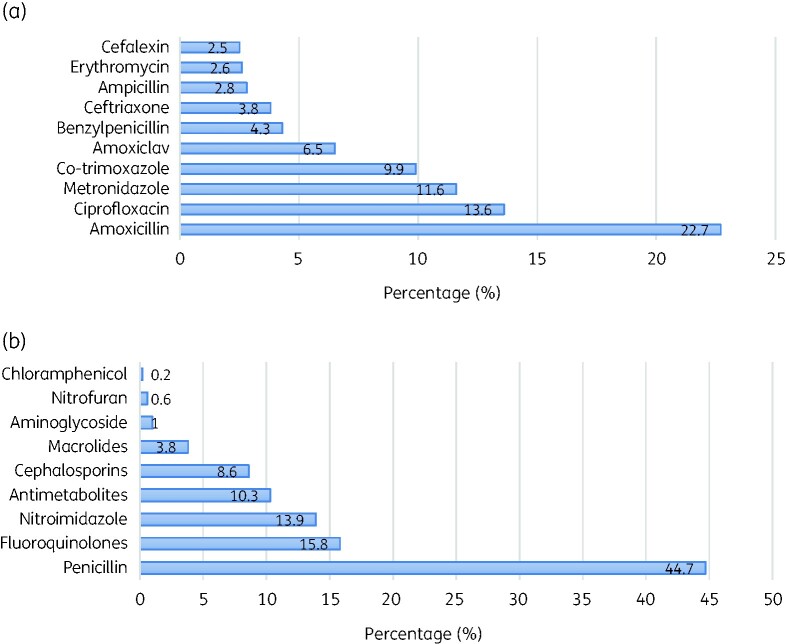
Out of 624 prescriptions containing antibiotics, amoxicillin was the most common (22.7%) followed by ciprofloxacin (13.6) then metronidazole (11.6%). In terms of classes, penicillins were the most prescribed antibiotics (44.7%) followed by fluoroquinolones (15.8%) (Figure 1).
Figure 1.
Commonly prescribed antibiotics (a) and their classes (b).
Class of antibiotics prescribed by conditions encountered
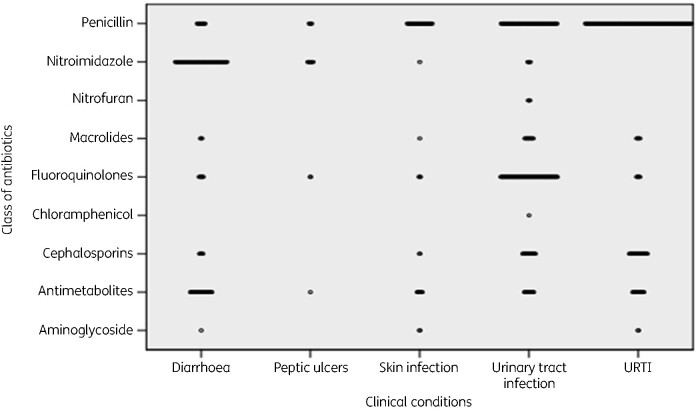
Penicillins were commonly used to treat URTI, nitroimidazoles for diarrhoea and fluoroquinolones for urinary tract infection. Aminoglycosides were rarely used for treatment diarrhoea, skin and URTI (Figure 2).
Figure 2.
Class of antibiotics prescribed by condition encountered.
Predictors for laboratory investigation in the suspected infected patients
The univariate analysis found that patients with peptic ulcers had a 4.6-fold higher chance of having laboratory investigation as compared with those patients with skin infections [crude odds ratio (cOR) = 4.6, 95% CI = 2.92–7.131, P = 0.0001], which was similar to the multivariate analysis [adjusted OR (aOR)=5.0, 95% CI = 3.1–8.126, P = 0.0001]. Public health centres were more likely to conduct laboratory investigations before antibiotic prescription as compared with private health care centres by univariate (cOR = 2.6, 95% CI = 1.691–3.709, P = 0.001) and multivariate analysis (aOR = 2.9, 95% CI: 1.887–4.512, P = 0.0001) (Table 3).
Table 3.
Predictors for laboratory investigation for the suspected infectious disease
| Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Categories | cOR | 95% CI | P valuea | aOR | 95% CI | P valuea |
| Age (years) | 5 | 0.9 | 0.343–2.08 | 0.713 | 1.2 | 0.423–3.41 | 0.73 |
| 5–14 | 1.9 | 0.73–4.90 | 0.187 | 2.1 | 0.709–6.264 | 0.18 | |
| 15–24 | 1.4 | 0.64–3.64 | 0.494 | 1.4 | 0.458–4.135 | 0.569 | |
| 25–44 | 1.3 | 0.52–3.25 | 0.575 | 1.1 | 0.371–3.072 | 0.903 | |
| 25–64 | 2.0 | 0.73–5.36 | 0.181 | 1.8 | 0.557–5.633 | 0.333 | |
| 65 | Ref | ||||||
| Sex | Male | 1.2 | 0.89–1.70 | 0.205 | 1.0 | 0.741–1.547 | 0.717 |
| Female | Ref | ||||||
| Payment method | User fee | 0.8 | 0.572–1.134 | 0.215 | 0.7 | 0.481–1.042 | 0.08 |
| Insurance | Ref | ||||||
| Disease condition | UTI | 0.9 | 0.614–1.499 | 0.959 | 1.0 | 0.647–1.657 | 0.885 |
| URTI | 3.4 | 1.045–11.01 | 0.042 | 2.6 | 0.779–8.96 | 0.119 | |
| Diarrhoea | 0.4 | 0.189–0.656 | 0.001 | 0.3 | 0.183–0.663 | 0.001 | |
| Peptic ulcers | 4.6 | 2.92–7.131 | 0.0001 | 5.0 | 3.1–8.126 | 0.0001 | |
| Skin infection | Ref | ||||||
| Hospital category | Public | 2.6 | 1.691–3.709 | 0.0001 | 2.9 | 1.887–4.512 | 0.0001 |
| Private | Ref | ||||||
Ref, reference category predicted the probability of laboratory investigation (yes); cOR, crude odds ratio; aOR, adjusted odds ratio; URTI, upper respiratory tract infection; UTI, urinary tract infection.
P values < 0.05 are shown in bold.
Factors associated with antibiotic prescription
Patients presented with peptic ulcers were 4.4-fold more likely to receive antibiotics as compared with those with skin infections (aOR = 4.4, 95% CI = 1.918–10.13, P = 0.0001). Compared with those with skin infections, patients with diarrhoea had 70% chance of not being prescribed antibiotics (aOR = 0.3, 95% CI = 0.133–0.489, P = 0.001) (Table 4).
Table 4.
Factor associated with antibiotics prescription
| Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Categories | cOR | 95% CI | P valuea | aOR | 95% CI | P valuea |
| Sex | Male | 1.3 | 0.75–2.039 | 0.315 | 1.8 | 0.933–3.325 | 0.081 |
| Female | Ref | ||||||
| Payment method | User fee | 0.8 | 0.494–1.322 | 0.215 | 0.7 | 0.416–1.177 | 0.178 |
| Insurance | Ref | ||||||
| Disease condition | UTI | 1.2 | 0.613–2.272 | 0.62 | 1.2 | 0.623–2.33 | 0.581 |
| URTI | 2.4 | 0.299–18.64 | 0.415 | 2.4 | 0.295–19.103 | 0.417 | |
| Diarrhoea | 0.3 | 0.16–0.555 | 0.0001 | 0.3 | 0.133–0.489 | 0.0001 | |
| Peptic ulcers | 3.8 | 1.687–8.529 | 0.001 | 4.4 | 1.918–10.130 | 0.0001 | |
| Skin infection | Ref | ||||||
| Hospital category | Public | 1.5 | 0.838–2.752 | 0.0001 | 1.8 | 0.933–3.325 | 0.081 |
| Private | Ref | ||||||
| Laboratory investigation | Yes | 1.3 | 0.810–2.079 | 0.278 | 0.7 | 0.416–1.177 | 0.178 |
| No | |||||||
Ref, reference category predicted the probability of laboratory investigation (yes); cOR, crude odds ratio; aOR, adjusted odds ratio; URTI, upper respiratory tract infection; UTI, urinary tract infection.
P values < 0.05 are shown in bold.
Factors associated with prescription of more than one antibiotic
Patients with diarrhoea were 2.6-fold more likely to be prescribed with more than one antibiotic as compared with those with skin infections by both univariate (cOR = 2.6, 95% CI = 1.239–5.451, P = 0.012) and multivariate analysis (aOR = 2.6, 95% CI = 1.206–5.491, P = 0.015) (Table 5).
Table 5.
Predictors for being prescribed with more than one antibiotic
| Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Categories | cOR | 95% CI | P value | aOR | 95% CI | P value |
| Age (years) | 5 | 0.9 | 0.3–2.555 | 0.807 | 1.0 | 0.334–3.038 | 0.990 |
| 5–14 | 0.4 | 0.13–1.387 | 0.187 | 0.5 | 0.142–1.605 | 0.232 | |
| 15–24 | 0.7 | 0.2–2.119 | 0.494 | 0.7 | 0.223–2.505 | 0.637 | |
| 25–44 | 0.7 | 0.238–2.17 | 0.575 | 0.8 | 0.261–2.537 | 0.723 | |
| 25–64 | 0.3 | 0.082–1.249 | 0.101 | 0.4 | 0.091–1.46 | 0.154 | |
| 65 | Ref | ||||||
| Sex | Male | 0.93 | 0.591–1.47 | 0.763 | 1.0 | 0.59–1.573 | 0.881 |
| Female | Ref | ||||||
| Payment method | User fee | 0.9 | 0.524–1.419 | 0.56 | 0.9 | 0.544–1510 | 0.705 |
| Insurance | Ref | ||||||
| Disease condition | UTI | 1.0 | 0.497–1.859 | 0.906 | 0.9 | 0.481–1.832 | 0.853 |
| URTI | 0.9 | 0/190–4.227 | 0.889 | 1.2 | 0.241–5.774 | 0.638 | |
| Diarrhoea | 2.6 | 1.239–5.451 | 0.012 | 2.6 | 1.206–5.491 | 0.015 | |
| Peptic ulcers | 1.0 | 0.582–1.827 | 0.916 | 1.1. | 0.585–2.051 | 0.777 | |
| Skin infection | Ref | ||||||
| Hospital category | Public | 0.6 | 0.349–1.088 | 0.095 | 0.589 | 0.324–1.071 | 0.082 |
| Private | Ref | ||||||
| Lab investigation | Yes | 0.7 | 0.469–1.165 | 0.194 | 1.2 | 0.65–2.397 | 0.506 |
| No | Ref | ||||||
| No. lab tests | None | 1.5 | 0.804–2.628 | 0.216 | – | – | – |
| One | 1.0 | 0.42–2.228 | 0.939 | 1.0 | 0.0406–2.273 | 0.927 | |
| Two | 1.3 | 0.608–2.717 | 0.510 | 1.4 | 0.644–2.974 | 0.406 | |
| Three | Ref | ||||||
Ref, reference category predicted the probability of laboratory investigation (yes); cOR, crude odds ratio; aOR, adjusted odds ratio; URTI, upper respiratory tract infection; UTI, urinary tract infection.
P values < 0.05 are shown in bold.
Discussion
We examined 604 encounters and found poor antibiotics prescription practice as compared to the WHO optimal values.11 The studied health centres in Ilala district had an average of 1.99 medicines prescribed per consultation; of those medicines, 51.9% were antibiotics. An average of 84% of the antibiotics was prescribed by its generic name. Additionally, these primary health facilities had an average of 97.6% of the antibiotics that appeared on the NEML, while 3.2% of the encounters were prescribed injections. These results were similar to the previous findings, which found a proportion of prescriptions with injections of 18.1% while those containing antibiotics was 67.7%, and the average number of medicines per prescription was 2.3.12
The proportion of medicines prescribed by generic name was 95.7%, while the proportion that contained medicines in line with the NEML was 96.7%.5 Except for prescribed injections, other core indicators exceeded the optimal values recommended by WHO,12 implying inappropriate prescriptions in the study area. A systematic review done by Ofori-Ansenso et al. 20166 indicated that the overall antibiotic prescription rate in Africa was 46.8%, whereas Tanzania had a 58% prescription rate. These previous findings were higher compared with our current study (51.9%). Nonetheless, both findings are still higher than the WHO targets6 but lower than those conducted elsewhere.13,14
We found a relatively high adherence level of 97.6% to the respective NEML recommendation,7 although this is inconsistent with the recommendation by WHO to fully (100%) adhere to the list. The situation is similar in other countries in the region. A study by Mashalla et al. 201710 in Botswana reported a 96.1% adherence. A constantly high prescription of antibiotics implies the increase of non-adherence to treatment guidelines within specified countries. A possible explanation could be that healthcare providers prescribe antibiotics despite their sound clinical judgement in meeting patients’ demands. Also, most antibiotics available at primary health facilities are used for the management of common and relatively uncomplicated infections. This may prompt physicians to seek alternative medicine not listed in NEML.7 Other factors such as patients, healthcare providers, the working environment, the drug companies, legal regulations, information and misinformation about medicines, and market forces could be associated with non-compliance with appropriate antibiotic use.11,12 These differences can sometimes be attributed to the differences in the study population, for example, the study by Atif et al.,11 was conducted at an Emergency Department.
The classes of antibiotics commonly prescribed were penicillins (amoxicillin), fluoroquinolones (ciprofloxacin), and nitroimidazoles (metronidazole) in that order. Antibiotic class prescription was examined in relation to the clinical conditions, and penicillin and fluoroquinolones were more commonly prescribed for urinary tract infections, and nitroimidazoles were frequently prescribed for diarrhoea. These were similar to a study conducted in Ethiopia14 where, penicillins 51.9% (amoxicillin: 44.8%) and fluoroquinolones 18.3% (ciprofloxacin: 13.6%) were most common. This similarity could be due to shared clinical conditions and treatment practices in tropical regions.
The prescriptions of antibiotics mostly complied with the NEML recommendation.7 It should be noted that the prescription of antibiotics in primary health care in Tanzania is not necessarily guided by bacterial culture and antimicrobial susceptibility testing results.7 Some guidelines, such as integrated management of childhood illness (IMCI) recommend no antibiotics for some conditions that are mostly viral in origin.15 Nevertheless, empirical treatment was frequently encountered, for example children with diarrhoea were empirically treated with cotrimoxazole and erythromycin. Patients with peptic ulcers were more likely to receive antibiotics, and those with diarrhoea had a higher chance of being prescribed more than one antibiotic. However, the question remains whether the diagnosis of peptic ulcers was correctly made, given that the primary health centres in Tanzania have limited laboratory capacity to perform microbiological tests. A follow-up qualitative study on why there is high prescription rate among patients with peptic ulcers is recommended.
Since primary health care centres in Ilala district, Tanzania rarely have inpatients, conclusions from this study are limited to outpatients. Four HCF were included with only one private HCF, given the possibility of site bias as a result of including only one private health care centre, interpretation of practice in private facilities should be done with care. We acknowledge the limitations based on the study design used, retrospective studies had a characteristic of having documentation and reporting bias. However, data collection ensured that all required data were collected accordingly. During data collection it was not clear what type of diarrhoea was documented (non-bloody diarrhoea or bloody diarrhoea), this poses potential limitations to the conclusion. Nonetheless, in managing diarrhoea antibiotics use is less prioritized.7
Conclusions
We found inappropriate prescriptions of antibiotics in the studied primary health care facilities in Ilala District. Factors such as disease condition strongly predicted the prescription practice. It was found that patients with peptic ulcers had higher chance of receiving antibiotics, which were rarely prescribed to patients with diarrhoea. However, patients with diarrhoea were more likely to receive at least one antibiotic. URTI was the commonly diagnosed infection, followed by urinary tract infection, then diarrhoea. Amoxicillin was the leading prescribed antibiotic, followed by ciprofloxacin then metronidazole. Antibiotics stewardship programmes should be extended to primary health care facilities in Ilala district. This will include prescription based on bacterial culture and susceptibility testing, formulating strategies for management of URTI, reducing inappropriate prescription for viral infection and adhering to national treatment guidelines. Lastly, a regionwide cross-sectional survey study is warranted.
Acknowledgements
We thank the medical officers in charge for granting permissions to collect data in their facilities. We acknowledge the cooperation received from the study site medical staff during the phase of data collection.
Funding
This study was carried out as part of our routine work.
Transparency declarations
None to declare.
References
- 1. Dellit TH. Summary of the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Infect Dis Clin Pract 2007; 15: 263–4. [DOI] [PubMed] [Google Scholar]
- 2. Camins BC, King MD, Wells JB. et al. Impact of an antimicrobial utilization program on antimicrobial use at a large teaching hospital a randomized controlled trial. Infect Control Hosp Epidemiol 2009; 30: 931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neel R. Multidrug resistance of isolates of Methicillin resistant Staphylococcus aureus (MRSA) in paper currency notes from Meat sellers in Tanga, Tanzania. Int J Life Sci Biotechnol Pharma Res 2012; 1: 8–14. [Google Scholar]
- 4. World Health Organization (WHO). WHO Report on Surveillance of Antibiotic Consumption. 2018. https://www.who.int/medicines/areas/rational_use/who-amr-amc-report-20181109.pdf.
- 5. Wordl Health Organization (WHO). How to Investigate Drug Use in Health Facilities. 1993. https://www.who.int/medicines/publications/how-to-investigate_drug-use/en/.
- 6. Ofori-Asensio R, Brhlikova P, Pollock AM.. Prescribing indicators at primary health care centers within the WHO African region: a systematic analysis (1995–2015). BMC Public Health 2016; 16: 724.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. United Republic of Tanzania (URT). Standard Treatment Guidelines and National Essential Medicines Lists. Vol 39, 2008. http://www.tzdpg.or.tz/fileadmin/documents/dpg_internal/dpg_working_groups_clusters/cluster_2/health/key_sector_documents/tanzania_key_health_documents/standard_treatment_guidelines__correct_final_use_this-1.pdf.
- 8. United Republic of Tanzania (URT). Dar Es Salaam Region Basic Demographic and Socio-Economic Profile. 2016. www.nbs.go.tz.
- 9. Dyar OJ, Beovic B, Vlahovic-Palcevski V. et al. How can we improve antibiotic prescribing in primary care? Expert Rev Anti Infect Ther 2016; 14: 403–13. [DOI] [PubMed] [Google Scholar]
- 10. Mashalla Y, Setlhare V, Massele A. et al. Assessment of prescribing practices at the primary healthcare facilities in Botswana with an emphasis on antibiotics: Findings and implications. Clin Pract 2017; 71: 1–10. [DOI] [PubMed] [Google Scholar]
- 11. Atif M, Azeem M, Sarwar MR. et al. WHO/INRUD prescribing indicators and prescribing trends of antibiotics in the Accident and Emergency Department of Bahawal Victoria Hospital, Pakistan. Springerplus 2016; 5: 1928.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Irunde H, Minzi O, Moshiro C.. Assessment of rational medicines prescribing in healthcare facilities in four regions of Tanzania. J Pharm Pract Community Med 2017; 3: 225–31. [Google Scholar]
- 13. Adisa R, Orherhe OM, Fakeye TO.. Evaluation of antibiotic prescriptions and use in under-five children in Ibadan, SouthWestern Nigeria. Afr Health Sci 2018; 18: 1189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Worku F, Tewahido D.. Retrospective Assessment of Antibiotics Prescribing at Public Primary Healthcare Facilities in Addis Ababa, Ethiopia. Interdisplinary Perspect Infect Dis 2018; 2018: 4323769.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moyo F, Chasela C, Brennan AT. et al. Treatment outcomes of HIV-positive patients on first-line antiretroviral therapy in private versus public HIV clinics in Johannesburg, South Africa. Clin Epidemiol 2016; 8: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]