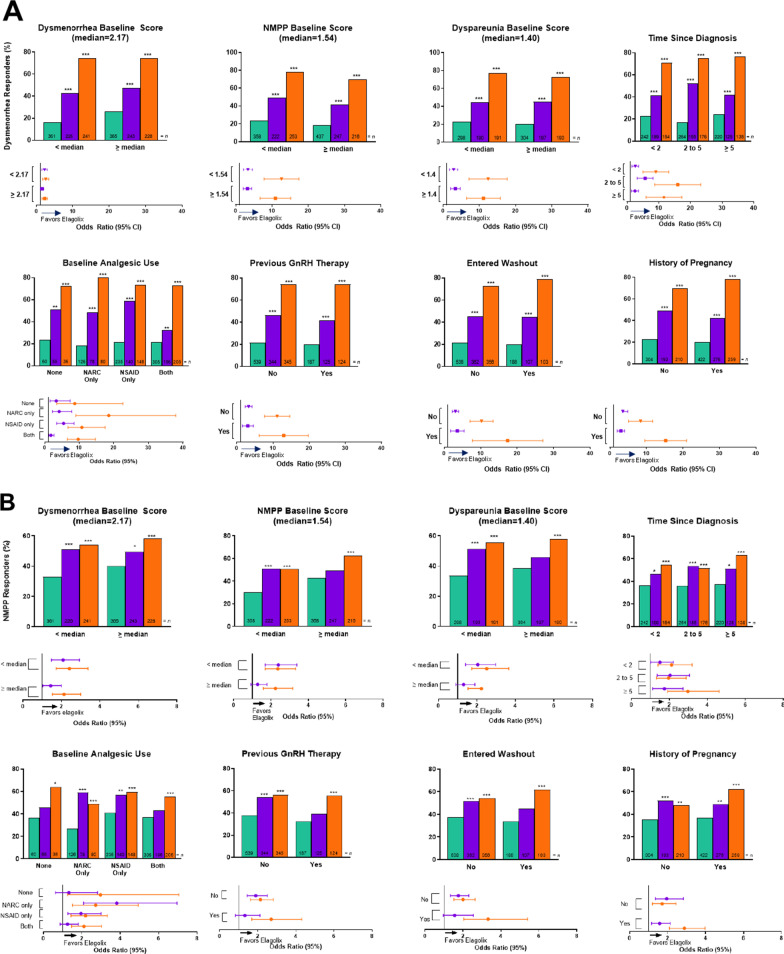
Fig. 2.
Co-primary endpoints at month 3 for integrated Elaris EM-I and Elaris EM-II by baseline disease severity subgroups. The treatment by subgroup interaction was significant (*p < 0.05, **p < 0.01). p values were obtained from a logistic regression model: responder/non-responder = base pain score + treatment + subgroup + treatment × subgroup for dysmenorrhea responders by previous analgesic use and history of pregnancy at baseline subgroups (A), and non-menstrual pelvic pain responders, non-menstrual pelvic pain baseline score, and history of pregnancy at baseline subgroups (B). Previous GnRH therapy includes both GnRH agonists and antagonists. Ratios equal the number of responders over the total number of women in each treatment group per subgroup. Green indicates placebo, purple indicates elagolix 150 mg QD, and orange indicates elagolix 200 mg BID. BID twice daily; DYS dysmenorrhea; DYSP dyspareunia; GnRH gonadotropin-releasing hormone; NARC narcotic/opioid; NMPP non-menstrual pelvic pain; NSAID non-steroidal anti-inflammatory drug; QD once daily