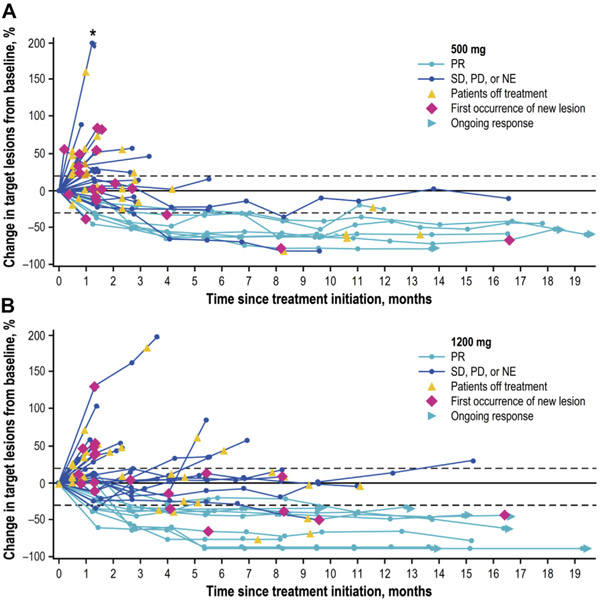
Figure 2.
Change from baseline in the sum of target lesion diameters over time according to RECIST 1.1 and as adjudicated by the IRC in patients treated with (A) 500 mg and (B) 1200 mg of bintrafusp alfa. IRC, independent review committee. NE, not evaluable; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease. *Patient had a greater than 200% increase in target lesion diameter from baseline.