Abstract
Mammographic breast density is a strong risk factor for breast cancer. We comprehensively investigated the associations of body mass index (BMI) change from ages 10, 18, and 30 to age at mammogram with mammographic breast density in postmenopausal women. We used multivariable linear regressions, adjusted for confounders, to investigate the associations of BMI change with volumetric percent density, dense volume, and non-dense volume, assessed using Volpara in 367 women. At the time of mammogram, the mean age was 57.9 years. Compared to women who had a BMI gain of 0.1-5 kg/m2 from age 10, women who had a BMI gain of 5.1-10 kg/m2 had a 24.4% decrease (95% confidence interval [95% CI], 6.0%-39.2%) in volumetric percent density; women who had a BMI gain of 10.1-15 kg/m2 had a 46.1% decrease (95%CI, 33.0%-56.7%) in volumetric percent density; and women who had a BMI gain of >15 kg/m2 had a 56.5% decrease (95%CI, 46.0%-65.0%) in volumetric percent density. Similar, but slightly attenuated associations were observed for BMI gain from ages 18 and 30 to age at mammogram and volumetric percent density. BMI gain over the life course was positively associated with non-dense volume, but not dense volume. We observed strong associations between BMI change over the life course and mammographic breast density. The inverse associations between early life adiposity change and volumetric percent density suggest that childhood adiposity may confer long-term protection against postmenopausal breast cancer via its effect of mammographic breast density.
Keywords: mammographic breast density, breast cancer, postmenopausal, adiposity, body mass index, BMI change, weight change
Introduction
High mammographic breast density is a well-established risk factor for breast cancer (1-4). Women with ≥ 75% mammographic breast density have a 4 to 6-fold increased risk of breast cancer than women with ≤ 5% mammographic breast density (5-7). The underlying mechanisms through which mammographic breast density increases breast cancer risk are, however, not well understood. Although mammographic breast density is highly heritable, it is also influenced by shared risk factors for breast cancer (8-10).
Obesity is associated with a higher risk of developing breast cancer in postmenopausal women, especially hormone receptor-positive disease (11-13). We recently showed that adiposity at age 18 was inversely associated with both premenopausal and postmenopausal breast cancer, and long-term weight gain from age 18 both during premenopause and postmenopause were associated with increased risk of postmenopausal breast cancer (14). In addition to being a risk factor, mammographic breast density is an intermediate phenotype for breast cancer (4); therefore it is possible that the associations of changes in adiposity over the life course with breast cancer risk is mediated through its associations with mammographic breast density (15). However, only a limited number of studies have investigated the association between long-term adiposity change and mammographic breast density in postmenopausal women, with conflicting results (16-18). While one study reported an inverse association between weight gain from age 18 and mammographic breast density (16), two other studies reported positive associations (17,18), hence, new studies are needed to clarify the associations of long-term adiposity change with mammographic brest density. Further, there is no data on the association of adiposity change since age 10 with mammographic breast density assessed in postmenopausal women, in spite of the fact that weight change since age 10 is strongly associated with breast cancer risk and may partly explain the association of weight change since age 18 with breast cancer risk (14).
We, therefore, comprehensively investigated the associations between adiposity change (both body mass index [BMI] and weight change) from childhood (age 10), late adolescence (age 18), and early adulthood (age 30) to postmenopausal age with volumetric measures of mammographic breast density in women free from breast cancer.
Materials and Methods
Study design and populations
We recruited 400 mid-life postmenopausal women undergoing annual screening mammogram at the Joanne Knight Breast Health Center, at Siteman Cancer Center at Washington University School of Medicine, St. Louis, MO, between October 2017 and September 2018.
Women were eligible to participate in the study if they met the following inclusion criteria: (i) aged 50-64 years, (ii) postmenopausal (iii) able to comply with all required study procedures and schedule, including provision of blood samples at the time of enrollment. Exclusion criteria were: (i) history of any cancer, including breast cancer; (ii) history of breast augmentation, reduction, or implants; (iii) history of selective estrogen receptor modulators or denosumab use over the previous 6 months. Postmenopause was defined using a modification of the National Comprehensive Cancer Network definition, which does not require measurement of serum hormone levels (19). A woman was considered postmenopausal if she had either a prior bilateral oophorectomy, was age 60 or older, or if under age 60, had been amenorrhoeic for at least 12 months.
Eligible participants were mailed study flyers by research coordinators two to four weeks in advance of screening mammography. On the day of their mammogram, each study participant completed a blood draw and questionnaires that ascertained breast cancer risk factors and potential determinants of mammographic breast density. We excluded 32 women with error messages when their raw mammogram images were converted to volumetric measures using Volpara, and 1 woman due to missing information on weight at mammogram; hence, our final study population consisted of 367 women. The study was approved by the institutional review boards of the Washington University School of Medicine. All study participants provided informed consent.
Adiposity measures and adiposity change
Weight at mammogram was measured in light clothing without shoes using the OMRON full body sensor body composition monitor and scale (model HBF-514C), which also estimated percentage body fat. Height was measured using a fixed stadiometer. Waist circumference (cm) was measured as the point midway between the costal margin and iliac crest in the mid-axillary line. Hip circumference (cm) was measured at the widest point around the greater trochanter. Weight at ages 18 and 30 were obtained from questionnaires provided by study participants. We calculated BMI at different ages (ages 18, 30 and age at mammogram) by dividing weight (kg) at each age with height (m) squared (kg/m2) at mammogram. Body size at age 10 was self-reported using the 9-level figure somatotype pictogram developed by Stunkard et al.(20). For this study, the Stunkard 9-level figure pictogram was categorized into 4 groups: (i) body size 1 or 2; (ii) body size 3 or 4; (iii) body size 5; and (iv) body size 6 or higher. We estimated BMI at age 10 using BMI and Stunkard pictograms, both provided at age 10, from the Growing Up Today Study (21,22). Since the Stunkard 9-level figure somatotype pictogram for girls in the Growing Up Today Study ranged from 1 to only 7, we did not compute BMI at age 10 for 10 women in our study, whose body sizes at age 10 were larger than 7 (8 or 9).
We derived BMI change trajectories for three time periods, (i) between BMI at mammogram and BMI at age 10, (ii) between BMI at mammogram and BMI at age 18, and (iii) between BMI at mammogram and BMI at age 30. We defined the categories of BMI change as: (i) BMI loss, (ii) BMI gain of 0.1 - 5 kg/m2, (iii) BMI gain of 5.1 - 10 kg/m2, (iv) BMI gain of 10.1 - 15 kg/m2, (v) BMI gain > 15 kg/m2. We derived weight change trajectories for two time periods: (i) between weight at mammogram and weight at age 18, and (ii) between weight at mammogram and weight at age 30. We had no data on weight at age 10, hence we did not evaluate weight change from age 10. We defined the categories of weight change as: (i) weight loss, (ii) weight gain of 0.1 - 10 kg, (iii) weight gain of 10.1 - 20 kg, (iv) weight gain of 20.1 - 30 kg, (v) weight gain > 30 kg.
Volumetric mammographic breast density measures
We used Volpara [version 1.5, (Matakina Technology Limited, Wellington, New Zealand)] to obtain automated, objective volumetric mammographic breast density measurements. Volpara measures volumetric percent density, dense volume, and nondense volume (23,24). Volumetric percent density values range from 0.5% to 34.5%. Compared with Breast Imaging Reporting and Data System (BIRADs) 5th edition, Volpara volumetric percent density translates to (i) < 3.5%; (ii) ≥ 3.5 and < 7.5%; (iii) ≥7.5 and < 15.5%; and (iv) ≥ 15.5%. Volpara measures have been validated in many studies (25,26), and are highly reproducible (within-breast density measurement standard deviation [SD] is 0.99% (27).
Statistical analysis
We calculated descriptive statistics, mean and SD for continuous variables and percentages for categorical variables as appropriate. We investigated age-adjusted correlations between adiposity measures and mammographic breast density measures using Spearman partial correlation coefficients (r). Next, we used multivariable linear regression models to evaluate the associations of adiposity change over the life course with postmenopausal mammographic breast density measures. Volumetric percent density, dense volume, and non-dense volume were all natural log transformed to ensure the normality of the residuals. The beta coefficients and 95% confidence intervals (CI) from the regression models were back-transformed to allow an easier interpretation of the results. The back-transformed beta coefficients are presented as percentage differences (Diff %), which is estimated as Diff% = (exp (β) − 1) ×100, and interpreted as a unit change in an adiposity measure associated with percent change in volumetric percent density, dense volume, or non-dense volume. We adjusted the multivariable linear regression models for age at mammogram (continuous, years), family history of breast cancer (yes/no), age at menarche (continuous, years), parity and age at first birth (categorical), race (Non-Hispanic white/African American/Others), current alcohol consumption (yes/no), ever use of menopausal hormone therapy (yes/no), and BMI at age 10 (continuous, kg/m2). These variables were selected as potential confounders a priori based on their established associations with mammographic breast density and/or breast cancer risk. In addition, they were statistically significant in the final multivariable regression models. We also evaluated the associations of adiposity change at various time periods and adiposity measures at time of mammogram (e.g. percentage body fat, hip and waist circumference) with mammographic breast density in multivariable linear regression models. We further stratified our analyses by race and use of menopausal hormone therapy. Interactions between variables (race, or menopausal hormone therapy) and BMI change over the life course were assessed by including cross-product terms (i.e. race × BMI change, menopausal hormone therapy × BMI change) in multivariable-adjusted models. The statistical significance of an interaction term was based on Wald tests. We used SAS statistical software (version 9.4; SAS Institute Inc) for analyses. All P values were two-sided and P < 0.05 was considered statistically significant.
Results
The mean age at the time of screening mammogram was 57.9 years (range, 50 - 65 years) (Table 1). The majority (62.1%) of participants were non-Hispanic White, and 35.4% were African American. The mean BMIs at different ages were: 17.2 kg/m2 (age 10), 21.8 kg/m2 (age 18), 25.1 kg/m2 (age 30), and 31.3 kg/m2 (age at mammogram). The mean volumetric percent density was 6.2% (range, 1.7% - 35%), the mean dense volume was 121.4 cm3 (range, 10.2 cm3 - 913.8 cm3), and the mean non-dense volume was 2136.8 cm3 (range, 143.9 cm3 - 12708.7 cm3).
Table 1.
Characteristics of 367 postmenopausal women recruited during annual screening mammogram at the Joanne Knight Breast Health Center, Washington University School of Medicine, St. Louis, MO
| Characteristics | N | Mean ± SD/ Percentage (%) |
|---|---|---|
| Age at mammogram (years) | 367 | 57.94 ± 3.82 |
| Age at menarche (years) a | 361 | 12.76 ± 1.70 |
| Parity and age at first birth b | ||
| Nulliparous | 63 | 17.17% |
| 1-2 children, < 25 years | 84 | 22.89% |
| 1-2 children, 25-29 years | 63 | 17.17% |
| 1-2 children, ≥ 30 years | 57 | 15.53% |
| ≥ 3 children, < 25 years | 63 | 17.17% |
| ≥ 3 children, ≥ 25 years | 33 | 8.99% |
| Ever breastfeed | 164 | 44.69% |
| Family history of breast cancer c | 91 | 24.80% |
| Ever used menopausal hormone therapy d | 122 | 33.24% |
| Race | ||
| Non-Hispanic White | 228 | 62.13% |
| African American | 130 | 35.42% |
| Others | 9 | 2.45% |
| Education e | ||
| High school or less than high school | 64 | 17.44% |
| Post high school training or some college | 106 | 28.88% |
| College graduate | 105 | 28.61% |
| Postgraduate | 90 | 24.52% |
| Current alcohol consumption f | 217 | 59.13% |
| Adiposity measures g | ||
| Body fat (%) h | 362 | 41.31 ± 8.64 |
| Hip circumference (cm) h | 365 | 110.09 ± 18.45 |
| Waist circumference (cm) h | 366 | 96.05 ± 18.39 |
| Height (cm) h | 367 | 163.51 ± 6.69 |
| BMI at age 10 years (kg/m2) i | 357 | 17.21 ± 2.99 |
| BMI at age 18 years (kg/m2) i | 367 | 21.80 ± 4.46 |
| BMI at age 30 years (kg/m2) i | 367 | 25.08 ± 5.90 |
| BMI at mammogram (kg/m2) i | 367 | 31.28 ± 7.70 |
| Weight at age 18 years (kg) | 367 | 58.05 ± 10.91 |
| Weight at age 30 years (kg) | 367 | 66.72 ± 14.43 |
| Weight at mammogram (kg) | 367 | 83.53 ± 20.55 |
| Mammographic density | ||
| Volumetric percent density (%) | 367 | 6.22 ± 4.15 |
| Dense volume (cm3) | 367 | 121.36 ± 125.22 |
| Non-dense volume (cm3) | 367 | 2136.76 ± 2064.92 |
Six women had missing information for age at menarche.
Four women had missing information for parity and age at first birth.
Six women had missing information for family history of breast cancer.
One woman had missing information for ever use of menopausal hormone therapy.
Two women had missing information for education.
Two women had missing information for current alcohol consumption.
Five women had missing information for body fat, two women had missing information for hip circumference, one woman had missing information for waist circumference, ten women had missing information for BMI at age 10.
Adiposity measures at the time of mammogram.
Body mass index (BMI) was calculated as weight (kg) at each age divided by height squared (m2).
The mean volumetric percent density by BMI change category, stratified by three time periods, are presented in Figure 1. From age 10 to age at mammogram, the mean volumetric percent density decreased from 10.5% to 4.4% among women who had a BMI gain of 0.1 - 5 kg/m2 among women who had a BMI gain >15 kg/m2 (Figure 1a). Similar decreases in the mean volumetric percent density were observed from ages 18 and 30 to age at mammogram (Figures 1b and 1c).
Figure 1. Mean volumetric percent density and 95% confidence interval (CI) of body mass index (BMI) changes over the life course among 367 postmenopausal women.
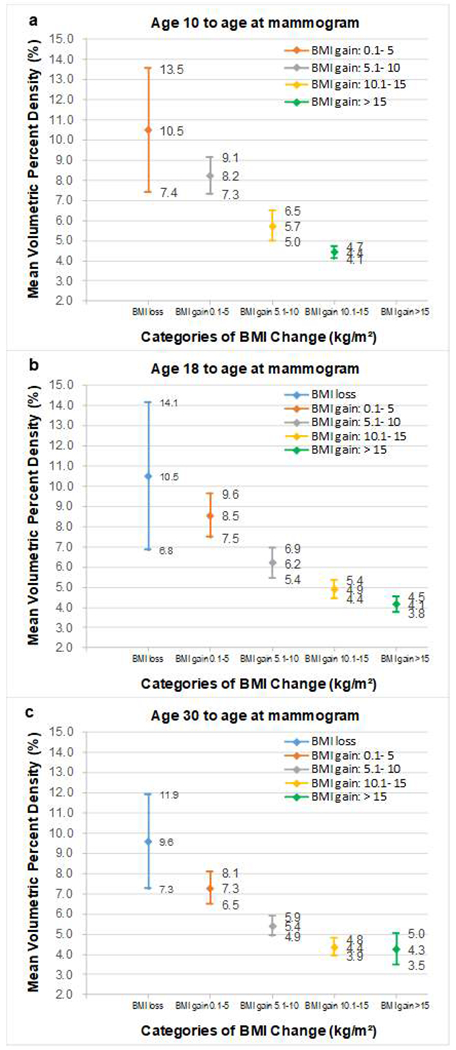
There were 356 women who had BMI gain from age 10 to age at mammogram, we did not show data on 1 woman who had BMI loss from age 10 to age at mammogram; 10 women had missing information for BMI from age 10 to age at mammogram.
Age-adjusted Spearman partial correlations between adiposity measures and mammographic breast density measures are summarized in Table 2. All adiposity measures (except for height) were significantly inversely correlated with volumetric percent density (r range, -0.18 to -0.59), with the strongest correlation for BMI at mammogram and weight at mammogram (r = -0.59) and positively correlated with non-dense volume (r range, 0.14 - 0.50), with the strongest correlation for weight at mammogram and hip circumference at mammogram (r = 0.50). We observed weaker positive correlations between adiposity measures and dense volume.
Table 2.
Partial spearman correlation coefficients between adiposity measures and mammographic breast density a
| Adiposity measures | N | Volumetric percent density (%) |
Dense volume (cm3) |
Non-dense volume (cm3) |
|---|---|---|---|---|
| Body fat (%) b | 362 | −0.54* | 0.21* | 0.44* |
| Hip circumference (cm) b | 365 | −0.52* | 0.26* | 0.50* |
| Waist circumference (cm) b | 366 | −0.57* | 0.22* | 0.49* |
| Height (cm) b | 367 | 0.00 | 0.01 | 0.03 |
| BMI at age 10 years (kg/m2) | 357 | −0.18* | 0.12* | 0.19* |
| BMI at age 18 years (kg/m2) | 367 | −0.20* | 0.08 | 0.14* |
| BMI at age 30 years (kg/m2) | 367 | −0.35* | 0.16* | 0.30* |
| BMI at mammogram (kg/m2) | 367 | −0.59* | 0.23* | 0.49* |
| BMI change from age 10 to age at mammogram (kg/m2) | 357 | −0.53* | 0.21* | 0.44* |
| BMI change from age 18 to age at mammogram (kg/m2) | 367 | −0.52* | 0.20* | 0.44* |
| BMI change from age 30 to age at mammogram (kg/m2) | 367 | −0.47* | 0.15* | 0.37* |
| Weight at age 18 years (kg) | 367 | −0.21* | 0.09 | 0.18* |
| Weight at age 30 years (kg) | 367 | −0.37* | 0.19* | 0.35* |
| Weight at mammogram (kg) | 367 | −0.59* | 0.24* | 0.50* |
| Weight change from age 18 to age at mammogram (kg) | 367 | −0.54* | 0.22* | 0.47* |
| Weight change from age 30 to age at mammogram (kg) | 367 | −0.48* | 0.16* | 0.39* |
All Spearman partial correlation models were adjusted for age at mammogram (continuous, years); Spearman partial correlation coefficient statistically significant at p-value < 0.05.
Adiposity measures at the time of mammogram.
BMI at age 10 was inversely associated with volumetric percent density. A 1 kg/m2 increase in BMI at age 10 was associated with a 2.3% lower (95% CI, 0.5% - 4.0%) volumetric percent density (p-value =0.01, Table 3). There were strong inverse associations between BMI changes during the life course and volumetric percent density (Table 3). The largest decrease in volumetric percent density were observed for BMI gain from age 10 to age at mammogram. Compared to women who had a BMI gain of 0.1 - 5 kg/m2 from age 10, women who had a BMI gain of 5.1 - 10 kg/m2 had a 24.4% decrease (95% CI, 6.0% - 39.2%) in volumetric percent density; women who had a BMI gain of 10.1 - 15 kg/m2 had a 46.1% (95% CI, 33.0% - 56.7%) decrease in volumetric percent density; and women who had a BMI gain of > 15 kg/m2 had a 56.5% decrease (95% CI, 46.0% - 65.0%) in volumetric percent density. A 1 kg/m2 increase in BMI change from age 10 to age at mammogram was associated with a 3.5% decrease (95% CI, 2.8% - 4.2%) in volumetric percent density (Supplementary Table 1). Similar, but slightly attenuated associations were observed for BMI changes from ages 18 and 30 and volumetric percent density (Table 3). Compared to women who had a BMI gain of 0.1 - 5 kg/m2, women who had a BMI gain > 15 kg/m2 from age 18 had a 46.2% decrease (95% CI, 37.2% - 53.9%), and those who had a BMI gain of >15 kg/m2 from age 30 had a 34.0% decrease (95% CI, 18.6% - 46.4%) in volumetric percent density. Women who had a reduction in BMI from age 30 had a 23.4% increase (95% CI, 3.6% - 47.1%) in volumetric percent density, compared to women who had a BMI gain of 0.1 - 5 kg/m2. BMI reduction from age 18 was associated with a 6.4% increase in volumetric percent density, but it was not statistically significant.
Table 3.
Multivariable-adjusted associations between changes in body mass index (BMI) over the life course and mammographic breast density
| BMI b | N (%) | Volumetric percent density (%) |
Dense volume (cm3) | Non-dense volume (cm3) | |||
|---|---|---|---|---|---|---|---|
| %Diffa | 95% CI | %Diffa | 95% CI | %Diffa | 95% CI | ||
| BMI at age 10 years (kg/m2) c | 357 (97.3%) | −2.3 | −4.0,−0.5 | 1.4 | −1.6, 4.4 | 3.4 | 0.2, 6.7 |
|
BMI change from age 10 to age at mammogram (kg/m2) d |
|||||||
| BMI gain: 0.1-5 | 19 (5.2%) | Ref | Ref | Ref | |||
| BMI gain: 5.1 - 10 | 99 (27.0%) | −24.4 | −39.2,−6.0 | −17.5 | −46.2,26.3 | 17.0 | −22.6,76.7 |
| BMI gain: 10.1 - 15 | 102 (27.8%) | −46.1 | −56.7,−33.0 | 6.2 | −30.7,62.9 | 124.4 | 48.4,239.5 |
| BMI gain: > 15 | 136 (37.1%) | −56.5 | −65.0,−46.0 | 10.7 | −27.4,68.7 | 174.6 | 82.6,313.1 |
|
BMI change from age 18 to age at mammogram (kg/m2) d |
|||||||
| BMI loss | 18 (4.9%) | 6.4 | −16.3,35.2 | −8.9 | −41.6,41.9 | −16.4 | −46.1,29.8 |
| BMI gain: 0.1-5 | 81 (22.1%) | Ref | Ref | Ref | |||
| BMI gain: 5.1 - 10 | 108 (29.4%) | −24.8 | −34.3,−14.0 | 15.4 | −10.1,48.1 | 64.2 | 28.3,110.3 |
| BMI gain: 10.1 - 15 | 96 (26.2%) | −38.3 | −46.2,−29.3 | 11.5 | −13.5,43.7 | 89.3 | 47.2,143.5 |
| BMI gain: > 15 | 64 (17.4%) | −46.2 | −53.9,−37.2 | 53.3 | 15.2,104.1 | 179.8 | 110.7,271.5 |
|
BMI change from age 30 to age at mammogram (kg/m2) d |
|||||||
| BMI loss | 36 (9.8%) | 23.4 | 3.6,47.1 | 26.0 | −8.4,73.3 | −12.5 | −36.8,21.1 |
| BMI gain: 0.1-5 | 129 (35.2%) | Ref | Ref | Ref | |||
| BMI gain: 5.1 - 10 | 119 (32.4%) | −23.0 | −31.6,−13.3 | 11.0 | −10.4,37.6 | 43.6 | 15.4,78.7 |
| BMI gain: 10.1 - 15 | 58 (15.8%) | −32.5 | −41.7,−21.8 | 25.2 | −4.1,63.3 | 79.6 | 36.9,135.6 |
| BMI gain: > 15 | 25 (6.8%) | −34.0 | −46.4,−18.6 | 43.8 | −1.6,109.9 | 118.5 | 48.4,221.7 |
Percentage difference (%Diff) represents one unit change in BMI associated with percent change in volumetric percent density, dense volume, and non-dense volume.
There were 357 women who had BMI at age 10, 10 women had missing information for BMI at age 10; there were 356 women who had BMI gain from age 10 to age at mammogram, we did not show data on 1 woman who had BMI loss from age 10 to age at mammogram; 10 women had missing information for BMI from age 10 to age at mammogram.
All models were adjusted for age at mammogram (continuous, years), family history of breast cancer (yes/no), age at menarche (continuous, years), parity and age at first birth (categorical), race (Non-Hispanic white/African American/Others), current alcohol consumption (yes/no) and menopausal hormone therapy use (yes/no).
All models were adjusted for age at mammogram (continuous, years), family history of breast cancer (yes/no), age at menarche (continuous, years), parity and age at first birth (categorical), race (Non-Hispanic white/African American/Others), current alcohol consumption (yes/no), menopausal hormone therapy use (yes/no), and BMI at age 10 (continuous, kg/m2).
BMI gain over the life course was positively associated with non-dense volume, but not dense volume (Table 3). Compared to women who had a BMI gain of 0.1 - 5 kg/m2 from age 10, women who had a BMI gain of 5.1 - 10 kg/m2 had a statistically non-significant 17.0% increase in non-dense volume; women who had a BMI gain of 10.1 - 15 kg/m2 had a 124.4% increase (95% CI, 48.4% - 239.5%) in non-dense volume; and women who had a BMI gain of > 15 kg/m2 had a 174.6% increase (95% CI, 82.6% - 313.1%) in non-dense volume. The associations of BMI changes from ages 18 and 30 to age at mammogram with non-dense volume were similar to those of BMI change from age 10. There were no consistent associations between BMI change over the life course and dense volume, though women who gained >15 BMI units after age 18 had a significant increase in dense volume.
The associations of weight change over the life course and mammographic breast density were very similar to what we observed for BMI change (Table 4).
Table 4.
Multivariable-adjusted associations between change in weight over the life course and mammographic breast density
| Weight changeb | N (%) | Volumetric percent density (%) |
Dense volume (cm3) | Non-dense volume (cm3) | |||
|---|---|---|---|---|---|---|---|
| %Diffa | 95% CI | %Diffa | 95% CI | %Diffa | 95% CI | ||
| Weight change from age 18 to age at mammogram (kg) | |||||||
| Weight loss | 15 (4.1%) | 19.4 | −8.3,55.4 | −0.4 | −39.7,64.3 | −18.6 | −50.1,32.9 |
| Weight gain: 0.1-10 | 55 (15.0%) | Ref | Ref | Ref | |||
| Weight gain: 10.1 - 20 | 75 (20.4%) | −17.1 | −29.0,−3.1 | 21.2 | −9.8,62.9 | 50.4 | 12.6,100.8 |
| Weight gain: 20.1 - 30 | 89 (24.3%) | −33.9 | −43.4,−22.8 | 19.4 | −11.1,60.4 | 96.5 | 47.3,162.3 |
| Weight gain: > 30 | 133 (36.2%) | −46.1 | −53.4,−37.6 | 41.8 | 7.4,87.2 | 169.9 | 105.6,254.2 |
| Weight change from age 30 to age at mammogram (kg) | |||||||
| Weight loss | 30 (8.2%) | 23.7 | 2.2,49.7 | 42.0 | −0.5,102.6 | −0.6 | −30.3,41.8 |
| Weight gain: 0.1-10 | 91 (24.8%) | Ref | Ref | Ref | |||
| Weight gain: 10.1 - 20 | 112 (30.5%) | −24.4 | −33.4,−14.2 | 18.8 | −6.2,50.4 | 67.6 | 32.4,112.1 |
| Weight gain: 20.1 - 30 | 77 (21.0%) | −33.5 | −42.3,−23.3 | 33.1 | 2.1,73.4 | 106.4 | 58.5,168.9 |
| Weight gain: > 30 | 57 (15.5%) | −41.9 | −50.2,−32.2 | 38.6 | 4.1,84.7 | 140.9 | 80.9,220.7 |
Percentage difference (%Diff) represents one unit change in weight associated with percent change in volumetric percent density, dense volume, and non-dense volume.
All models were adjusted for age at mammogram (continuous, years), family history of breast cancer (yes/no), age at menarche (continuous, years), parity and age at first birth (categorical), race (Non-Hispanic white/African American/Others), current alcohol consumption (yes/no), menopausal hormone therapy use (yes/no) and for BMI at age 10 (continuous, kg/m2).
We further examined the associations between adiposity change over the life course and volumetric percent density stratified by race (Supplementary Table 2) and menopausal hormone therapy (Supplementary Table 3). We observed no interaction by race or by menopausal hormone therapy.
Discussion
BMI (and weight) gains after ages 10, 18, and 30 were inversely associated with volumetric percent density, while BMI (and weight) loss after age 30 was positively associated with volumetric percent density in postmenopausal women. Associations did not differ by race or menopausal hormone therapy use. To the best of our knowledge, our study is the first to comprehensively investigate the associations of adiposity change over the life course with volumetric measures of mammographic breast density in postmenopausal women.
The biological mechanisms through which adiposity change over the life course influences mammographic breast density in postmenopausal women are not well known, and the relation between adiposity and breast density features is complex, which is also complicated by dynamic changes in the body and breast composition over time (28). Adipose tissue is the largest endocrine organ in the body and the main source of peripheral estrogens, especially in postmenopausal women (29), thus adiposity change could play a role in hormone homeostasis throughout life (30). Increased childhood or adolescent adiposity modulate hormonal exposure (e.g. estrogen) and growth factor levels, thus influence breast cellular proliferation and breast density tissue development later in life (31-34). Progesterone signaling and, more recently, stem cell biology have also been shown to be associated with mammographic breast density. Progesterone increases breast density, and breast cancer incidence, independent of estrogen (35,36). A recent study showed that the expression of stem cell markers was increased in dense breast tissue as compared to non-dense breast tissue within the same woman (37).
The inverse associations we observed suggest that childhood adiposity may confer long-term protection on breast cancer risk, probably, via its effect on breast density. Childhood adiposity is inversely associated with the risk of both pre- and postmenopausal breast cancer, suggesting a long-term protective effect of adiposity at young ages on breast cancer risk later in life (38). This is in contrast with the higher risk of breast cancer in postmenopausal women who have greater body adiposity in adulthood (11-13). Early life, including childhood and adolescence, is a critical window for breast development (14). The rapid growth of breast tissue during this period, together with changing hormonal milieu, may influence mammographic breast density later in life and future breast cancer development (39,40).
Our results are similar to those from the Nurses’ Health Study where the authors reported an inverse association between weight gain after age 18 and percent density after adjusting for BMI at age 18 (beta coefficient range, -0.029 to -0.032, for the difference in percent mammographic breast density for every unit weight) (16). They did not, however, report on the associations of BMI change since age 10 and mammographic breast density, hence, we provide novel data on adiposity change since age 10 and mammographic breast density. Another longitudinal study demonstrated that a short-term increase in BMI over a 2 year period was associated with a decrease in percent dense volume (28). In contrast, two other studies reported that weight gain since age 18 was positively associated with percent breast density in postmenopausal women (17,18).These two studies, did not, however, adjust their analyses for early life weight, but adjusted for current weight, which likely accounts for the differences in results. We previously observed that adiposity measures at mammogram were correlated with adult weight gain in premenopausal women, thus, including the adiposity measure at mammogram in the regression models could lead to multicollinearity (41). We adjusted for BMI at age 10 instead of BMI at mammogram to take into consideration an individual’s initial body size, which likely explains why our findings are consistent with those of Nurses’ Health Study, that also adjusted for BMI at age 18 (16).
Our study has several strengths. Study participants were recruited among women attending annual routine screening mammogram, which enhances generalizability. We assessed mammographic breast density using Volpara, which provides robust volumetric measures of density, and is highly reproducible (42,43). Furthermore, our analytic approach is strengthened by controlling for important confounders, including adiposity at age 10.
Our study has limitations. This is a cross-sectional study, and body size at age 10 and weight at ages 18 and 30 were collected retrospectively. However, studies have demonstrated that there is a high correlation between individuals’ adult-recalled body size at earlier life and their measured BMI at earlier life (44,45).
In conclusion, changes in BMI and weight from childhood, late adolescence, and early adulthood were associated with mammographic breast density in postmenopausal women. The inverse associations between early life adiposity change and volumetric percent density suggest that childhood adiposity may confer long-term protection against breast cancer via its effect of mammographic breast density. There is a need to better understand how long-term adiposity changes, especially since age 10 is associated with mammographic breast density, as this could have utility in breast cancer prevention.
Supplementary Material
Acknowledgements
Funding:
The study is supported by funds from the Susan G. Komen Foundation (CCR15332379 - A.T. Toriola), NIH/NCI (R21CA216515 and R37CA235602 - A.T. Toriola), Siteman Cancer Center Siteman Investment Program. GAC is supported by Breast Cancer Research Foundation. The funders had no role in study design, data collection, analysis, interpretation of data, preparation of the report, or decision to publish. All authors had full access to all the data and analyses and had final responsibility for the decision to submit for publication.
Footnotes
Conflict of interest:
The authors declare no potential conflicts of interest.
References
- 1.Pettersson A, Graff RE, Ursin G, Santos Silva ID, McCormack V, Baglietto L, et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. Journal of the National Cancer Institute 2014;106(5) doi 10.1093/jnci/dju078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd NF, Lockwood GA, Martin LJ, Knight JA, Byng JW, Yaffe MJ, et al. Mammographic densities and breast cancer risk. Breast disease 1998;10(3-4):113–26. [DOI] [PubMed] [Google Scholar]
- 3.Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, et al. Mammographic features and breast cancer risk: effects with time, age, and menopause status. Journal of the National Cancer Institute 1995;87(21):1622–9 doi 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 4.Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, et al. Mammographic breast density as an intermediate phenotype for breast cancer. The Lancet Oncology 2005;6(10):798–808 doi 10.1016/s1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 5.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2006;15(6):1159–69 doi 10.1158/1055-9965.epi-06-0034. [DOI] [PubMed] [Google Scholar]
- 6.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. The New England journal of medicine 2007;356(3):227–36 doi 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 7.Ursin G, Ma H, Wu AH, Bernstein L, Salane M, Parisky YR, et al. Mammographic density and breast cancer in three ethnic groups. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2003;12(4):332–8. [PubMed] [Google Scholar]
- 8.Ursin G, Lillie EO, Lee E, Cockburn M, Schork NJ, Cozen W, et al. The relative importance of genetics and environment on mammographic density. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2009;18(1):102–12 doi 10.1158/1055-9965.Epi-07-2857. [DOI] [PubMed] [Google Scholar]
- 9.Douglas JA, Roy-Gagnon MH, Zhou C, Mitchell BD, Shuldiner AR, Chan HP, et al. Mammographic breast density--evidence for genetic correlations with established breast cancer risk factors. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2008;17(12):3509–16 doi 10.1158/1055-9965.Epi-08-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazari SS, Mukherjee P. An overview of mammographic density and its association with breast cancer. Breast Cancer 2018;25(3):259–67 doi 10.1007/s12282-018-0857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England journal of medicine 2003;348(17):1625–38 doi 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 12.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin 2017;67(5):378–97 doi 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report 2018. <https://www.wcrf.org/dietandcancer>.
- 14.Rosner B, Eliassen AH, Toriola AT, Che WY, Hankinson SE, Willett WC, et al. Weight and weight changes in early adulthood and later breast cancer risk. International journal of cancer 2017;140(9):2003–14 doi 10.1002/ijc.30627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen ZJ, Baker JL, Bihrmann K, Vejborg I, Sorensen TI, Lynge E. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast cancer research : BCR 2014;16(1):R4 doi 10.1186/bcr3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samimi G, Colditz GA, Baer HJ, Tamimi RM. Measures of energy balance and mammographic density in the Nurses’ Health Study. Breast cancer research and treatment 2008;109(1):113–22 doi 10.1007/s10549-007-9631-7. [DOI] [PubMed] [Google Scholar]
- 17.Pollan M, Lope V, Miranda-Garcia J, Garcia M, Casanova F, Sanchez-Contador C, et al. Adult weight gain, fat distribution and mammographic density in Spanish pre- and post-menopausal women (DDM-Spain). Breast cancer research and treatment 2012;134(2):823–38 doi 10.1007/s10549-012-2108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soguel L, Diorio C. Anthropometric factors, adult weight gain, and mammographic features. Cancer causes & control : CCC 2016;27(3):333–40 doi 10.1007/s10552-015-0706-1. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Breast Cancer; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis 1983;60:115–20. [PubMed] [Google Scholar]
- 21.Berkey CS, Rockett HR, Field AE, Gillman MW, Frazier AL, Camargo CA Jr., et al. Activity, dietary intake, and weight changes in a longitudinal study of preadolescent and adolescent boys and girls. Pediatrics 2000;105(4):E56 doi 10.1542/peds.105.4.e56. [DOI] [PubMed] [Google Scholar]
- 22.Alimujiang A, Imm KR, Appleton CM, Colditz GA, Berkey CS, Toriola AT. Adiposity at Age 10 and Mammographic Density among Premenopausal Women. Cancer prevention research (Philadelphia, Pa) 2018;11(5):287–94 doi 10.1158/1940-6207.Capr-17-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HN, Sohn YM, Han KH. Comparison of mammographic density estimation by Volpara software with radiologists' visual assessment: analysis of clinical-radiologic factors affecting discrepancy between them. Acta radiologica (Stockholm, Sweden : 1987) 2015;56(9):1061–8 doi 10.1177/0284185114554674. [DOI] [PubMed] [Google Scholar]
- 24.Ellison-Loschmann L, McKenzie F, Highnam R, Cave A, Walker J, Jeffreys M. Age and ethnic differences in volumetric breast density in new zealand women: a cross-sectional study. PloS one 2013;8(7):e70217 doi 10.1371/journal.pone.0070217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gubern-Merida A, Kallenberg M, Platel B, Mann RM, Marti R, Karssemeijer N. Volumetric breast density estimation from full-field digital mammograms: a validation study. PloS one 2014;9(1):e85952 doi 10.1371/journal.pone.0085952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Azziz A, Fan B, Malkov S, Klifa C, Newitt D, et al. Agreement of mammographic measures of volumetric breast density to MRI. PloS one 2013;8(12):e81653 doi 10.1371/journal.pone.0081653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonzo-Proulx O, Mawdsley GE, Patrie JT, Yaffe MJ, Harvey JA. Reliability of automated breast density measurements. Radiology 2015;275(2):366–76 doi 10.1148/radiol.15141686. [DOI] [PubMed] [Google Scholar]
- 28.Hart V, Reeves KW, Sturgeon SR, Reich NG, Sievert LL, Kerlikowske K, et al. The effect of change in body mass index on volumetric measures of mammographic density. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2015;24(11):1724–30 doi 10.1158/1055-9965.Epi-15-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liedtke S, Schmidt ME, Vrieling A, Lukanova A, Becker S, Kaaks R, et al. Postmenopausal sex hormones in relation to body fat distribution. Obesity (Silver Spring, Md) 2012;20(5):1088–95 doi 10.1038/oby.2011.383. [DOI] [PubMed] [Google Scholar]
- 30.Partain N, Mokdad A, Puzziferri N, Porembka J, Seiler S, Christie A, et al. Mammographic density changes in surgical weight loss-an indication for personalized screening. BMC Med Imaging 2018;18(1):10 doi 10.1186/s12880-017-0242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, et al. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2001;10(3):243–8. [PubMed] [Google Scholar]
- 32.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast cancer research : BCR 2008;10(1):201 doi 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houghton LC, Sisti JS, Hankinson SE, Xie J, Xu X, Hoover RN, et al. Estrogen Metabolism in Premenopausal Women Is Related to Early Life Body Fatness. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2018;27(5):585–93 doi 10.1158/1055-9965.Epi-17-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yochum L, Tamimi RM, Hankinson SE. Birthweight, early life body size and adult mammographic density: a review of epidemiologic studies. Cancer causes & control : CCC 2014;25(10):1247–59 doi 10.1007/s10552-014-0432-0. [DOI] [PubMed] [Google Scholar]
- 35.Anderson E The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 2002;4(5):197–201 doi 10.1186/bcr452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab 1999;84(12):4559–65 doi 10.1210/jcem.84.12.6194. [DOI] [PubMed] [Google Scholar]
- 37.Yaghjyan L, Stoll E, Ghosh K, Scott CG, Jensen MR, Brandt KR, et al. Tissue-based associations of mammographic breast density with breast stem cell markers. Breast Cancer Res 2017;19(1):100 doi 10.1186/s13058-017-0889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. American journal of epidemiology 2010;171(11):1183–94 doi 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su X, Hankinson SE, Clevenger CV, Eliassen AH, Tworoger SS. Energy balance, early life body size, and plasma prolactin levels in postmenopausal women. Cancer causes & control : CCC 2009;20(2):253–62 doi 10.1007/s10552-008-9240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hankinson SE, Colditz GA, Hunter DJ, Manson JE, Willett WC, Stampfer MJ, et al. Reproductive factors and family history of breast cancer in relation to plasma estrogen and prolactin levels in postmenopausal women in the Nurses’ Health Study (United States). Cancer causes & control : CCC 1995;6(3):217–24 doi 10.1007/bf00051793. [DOI] [PubMed] [Google Scholar]
- 41.Alimujiang A, Appleton C, Colditz GA, Toriola AT. Adiposity during early adulthood, changes in adiposity during adulthood, attained adiposity, and mammographic density among premenopausal women. Breast cancer research and treatment 2017;166(1):197–206 doi 10.1007/s10549-017-4384-4. [DOI] [PubMed] [Google Scholar]
- 42.Harvey JA, Stukenborg GJ, Cohn WF, Repich KL, Alonzo O, Novicoff WM, et al. Abstract P6-09-04: Volumetric breast density improves breast cancer risk prediction. Cancer research 2015;75(9 Supplement):P6-09-4-P6--4 doi 10.1158/1538-7445.Sabcs14-p6-09-04. [DOI] [Google Scholar]
- 43.Holland K, van Zelst J, den Heeten GJ, Imhof-Tas M, Mann RM, van Gils CH, et al. Consistency of breast density categories in serial screening mammograms: A comparison between automated and human assessment. Breast (Edinburgh, Scotland) 2016;29:49–54 doi 10.1016/j.breast.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 44.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19(8):570–2. [PubMed] [Google Scholar]
- 45.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. American journal of epidemiology 1993;138(1):56–64 doi 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


