Abstract
Lead (Pb), mercury (Hg) and fluoride (F) exposure during childhood is of concern owing to their toxicity. Also, evidence suggests that high and low exposure levels to manganese (Mn) and selenium (Se) during this vulnerable period are associated with an increased risk of adverse health effects. A reduced growth is associated with high Pb and F exposure; however, little is known about their impact on children’s body size, and there is a lack of consensus on the effects of Hg, Mn, and Se exposure on children’s anthropometric measures. This is particularly true for childhood metal co-exposures at levels relevant to the general population. We investigated the joint effects of exposure to a metal mixture (Pb, Mn, Hg, and Se in blood and F in plasma) on 6-11-year-old US children’s anthropometry (n = 1,634). Median F, Pb, Mn, Hg, and Se concentrations were 0.3 μmol/L, 0.5 μg/dL, 10.2 μg/L, 0.3 μg/L, and 178.0 μg/L, respectively. The joint effects of the five metals were modeled using Bayesian kernel machine and linear regressions. Pb and Mn showed opposite directions of associations with all outcome measured, where Pb was inversely associated with anthropometry. For body mass index and waist circumference, the effect estimates for Pb and Mn appeared stronger at high and low concentrations of the other metals of the mixture, respectively. Our findings suggest that metal co-exposures may influence children’s body mass and linear growth indicators, and that such relations may differ by the exposure levels of the components of the metal mixture.
Keywords: NHANES, childhood exposure, metal mixture, growth, body size
1. Introduction
Heavy metals pose a threat to human health because they are non-biodegradable and can be deposited in body tissues or organs to produce harm after initial exposure (Al Osman et al., 2019). Children are especially vulnerable because of their rapid growth and still developing detoxification mechanisms (Rodríguez-Barranco et al., 2013). Indicators of growth and body size in school-age children (e.g., height, weight, and waist circumference) play a role in school achievement (Crooks, 1995), and physical and emotional health in childhood and adolescence (Gelander, 2006). In addition to lifestyle factors (e.g., diet or physical activity), there has been interest in understanding the effect of environmental exposures on children’s anthropometric measures.
A number of studies have examined whether exposure to trace elements (hereafter, “metals”) affect children’s body size and growth, but the effects at exposure levels relevant to the general population and co-exposures to metal mixtures remain poorly understood. Generally, the primary sources of metal exposure include ingestion through drinking water or diet, inhalation, and dermal contact (Buckley et al., 2020). Existing studies have investigated prenatal exposures and their effects on body size in utero, at birth or in early life, as well as postnatal exposure on growth throughout childhood. For postnatal exposures, lead (Pb) has received the most attention, with consistent evidence that higher Pb exposure associates with shorter stature (Burns et al., 2017; Deierlein et al., 2019; Kerr et al., 2019; Raihan et al., 2018; Yang et al., 2013; Zeng et al., 2019) and may reduce body weight indicators across a wide range of ages (Cassidy-Bushrow et al., 2016; Deierlein et al., 2019; Kerr et al., 2019; Scinicariello et al., 2013; Zeng et al., 2019). Fewer studies have been done on mercury (Hg), with two studies suggesting positive associations with Body Mass Index (BMI) in 0-10-year-old children (Benefice et al., 2008; Gao et al., 2018). In contrast, another study found no association with Hg among children without permanent housing (Fábelová et al., 2018). High fluoride (F) exposure appears to negatively affect children’s height (Wang et al., 2007). Manganese (Mn) and Selenium (Se) are essential nutrients and as such are necessary for proper growth and development (Lewicka et al., 2017). Available evidence suggests that Mn and Se follow a U-shape dose-response curve with adverse effects at low and high exposure levels on birth outcomes and BMI among adults and young children (Ortega et al., 2013; Rayman, 2012; Signes-Pastor et al., 2019; Wang et al., 2016). To date, nearly all studies considered exposures to a single metal at a time.
Exposure to toxic and nutrient metals occur simultaneously as a mixture in real-life scenarios (Valeri et al., 2017); however, there has been little investigation of metal co-exposure effects on body size and growth during childhood. Three prior studies on postnatal exposures and children’s growth examined more than two metals (Fábelová et al., 2018; Gardner et al., 2013; Zeng et al., 2019). Measurement of prenatal exposure to multiple metals has been more common (Bloom et al., 2015; Freire et al., 2019; Gleason et al., 2016; Govarts et al., 2016; Sabra et al., 2017; Thomas et al., 2015); yet none of these studies assessed the effects of metal mixtures.
To better understand the potential risks associated with metal co-exposures at levels relevant to the general population and child anthropometry, we investigated the associations between blood concentration of metals, of nutritional (i.e., Mn and Se) and toxicological interest (i.e., F, Hg, and Pb), on body size and growth measurements of 6–11-year-old US children included in the 2013-16 National Health and Nutrition Examination Survey (NHANES).
2. Material and methods
2.1. Study Design and Population.
Participants were part of the NHANES, an ongoing cross-sectional survey conducted by the National Center for Health Statistics at the Centers for Disease Control and Prevention (CDC). The NHANES aims to assess the health and nutritional status of the civilian, non-institutionalized population in the US. Participants are selected into NHANES using a stratified, multistage probability sampling strategy based on selection of counties, blocks, households, and individuals in the households. Details of recruitment and design are available on the NHANES website (NHANES, 2016a). Procedures for the NHANES have been approved by the National Center for Health Statistics Research Ethics Review Board. Our current analyses included 6-11-year-old children participating in the 2013-14 and 2015-16 NHANES cycles.
2.2. Whole blood lead, manganese, total mercury, and selenium.
Venous whole blood samples were collected by phlebotomists; Pb, Mn, Hg, and Se levels were measured using an Inductively Coupled Plasma Mass Spectrometer with Dynamic Reaction Cell Technology (ELAN® DRC II) at the National Center for Environmental Health. Detailed methods of these procedures are published elsewhere (NHANES, 2015a, 2013a). The lower limit of detection (LOD) was 0.07 μg/dL for Pb, 0.99 μg/L for Mn, 0.28 μg/L for Hg, and 24.48 μg/L for Se for the 2013-16 study cycles (NHANES, 2018, 2016b, 2015a, 2013a). There were no observations below the LOD for Mn and Se. For Pb, only one observation was below the LOD. For Hg, 639 (39%) observations were below the LOD and they were evenly distributed by sex. Values below the LOD were entered as the LOD divided by the square root of two and included in statistical analyses (Lubin et al., 2004; Schisterman et al., 2006).
2.3. Plasma fluoride.
Concentrations of F from plasma samples were measured at the College of Dental Medicine, Georgia Regents University, Augusta, GA, using an ion-specific electrode. The LOD for this method is ~1 μmol/L (0.019 mg/L), which is usually higher than plasma F concentrations. To overcome this, the hexamethyldisiloxane facilitated diffusion method is applied, to transfer F from the plasma samples into an alkaline trapping solution of smaller volume. Details of these methods have been published previously (NHANES, 2015b, 2013b). There were no plasma F concentrations below the LOD.
2.4. Body measures.
Body measures of interest, such as weight (kg), waist circumference (cm), upper arm length (cm), and standing height (cm) were assessed in the Mobile Examination Center by health technicians. Children’s BMI (kg/m2) was calculated from weight and height measurements. Arm length was typically measured on the right side of the body, unless participants had a medical condition, amputation, or a cast on the right side. Weight was measured using a digital scale in kg. Detailed protocols have been previously published (NHANES, 2016a, 2013c).
2.5. Covariates.
Demographic details for the children, such as age, sex, and race were provided by a proxy in an interview. Socioeconomic status was measured as poverty-to-income ratio (PIR). Total caloric intake (kcal) was calculated using a single 24-hour dietary recall. An interviewer-administered questionnaire asked about the number of people in the household who smoked tobacco. Smokers in the household (i.e., household members smokers ≥1) was used as a proxy for secondhand smoke. Physical activity was measured through an interviewer-administered questionnaire at the participants’ home using the Computer Assisted Personal Interview system. Children reported whether or not they were engaged in physical activities outside of school in the preceding seven days. They were asked about practicing common sports such as basketball, baseball, bike riding, dancing, etc. A total of thirty-three activities were queried. Then, similar questions were asked about 22 activities at school. For the current analysis, participants received a score of one for each activity they reported and zero otherwise. Two separate scores were calculated by summing the positive responses for the outside-of-school and school-based physical activities.
2.6. Statistical analyses.
For all statistical analyses, we excluded participants with missing values for the covariates. The Spearman’s coefficients (ρ) were calculated for each metal pair and for anthropometric measurements. BMI, standing height, waist circumference, and upper arm length were approximately normally distributed. Plasma F and blood Pb, Mn, Hg, and Se concentrations were positively skewed. Covariate selection was based on prior studies as well as directed acyclic graphs using the DAGitty software; total calorie intake (kcal, continuous), race (Mexican American, non-Hispanic white, non-Hispanic black or another race, categorical), PIR (≤1.49 (the median) vs. >1.49), children’s age (years, continuous), smoker/s in the household (yes vs. no), children’s sex (boys vs. girls), and outside-of-school and school-based physical activity scores (continuous) were included as potential confounders. The two physical activity scores were uncorrelated (Spearman’s ρ = 0.10).
Bayesian kernel machine regression (BKMR) was performed primarily as an exploratory method to investigate interactions and joint effects of the five metals using the R package “bkmr” (Bobb et al., 2018, 2015). BKMR models were applied as Yi = h(Fi, Mni, Pbi, Sei, Hgi) + ßT Zi + ei, where Y is the continuous outcome of interest (i.e., BMI, standing height, waist circumference or upper arm length); h() is an exposure-response function that accommodates nonlinearity and interactions among mixture components: F, Pb, Mn, Hg, and Se concentrations natural log-transformed, centered and scaled; Z are the selected covariates and ß are the corresponding regression coefficients. All models included 10,000 Markov chain Monte Carlo iterations using the Gaussian kernel, with 5,000 used as burn-in. The BKMR model is not able to accommodate sample weights yet, and thus we used unweighted estimation; however, all our models included several covariates that are used to calculate the weights (e.g., age, sex, or race) (Kim et al., 2017; Zhang et al., 2019).
Linear regression analyses were then conducted to further evaluate and quantify the associations between metal exposure and children’s anthropometric measures. The F, Pb, Mn, Hg, and Se concentrations were log-transformed to approximate normal distribution and divided by their interquartile range (IQR) for normalization before inclusion in the regression models. Anthropometric measurements (i.e., BMI, standing height, waist circumference, and upper arm length) were entered in the models as dependent variables. A threshold of α = 0.05 was used to define associations as statistically significant. We first conducted analyses in the overall sample (adjusted for sex), then stratified by sex. All analyses and graphics were performed using R version 3.5.1 (R Core Team, 2014).
3. Results
3.1. Study population characteristics.
Out of 2,703 children included in the 2013-16 NHANES cycles, 2,092 had blood samples analyzed for F, Pb, Mn, Hg and Se concentrations. Of those, 2,049 had complete information on body size and growth parameters (i.e., BMI, standing height, waist circumference, and upper arm length). Finally, 1,634 children, evenly distributed across cycles (n = 835 and n = 799 in 2013-14 and 2015-16, respectively) and sex (n = 839 boys and n = 795 girls), had complete data on the covariates and were included in the statistical analyses (Figure S1). The biochemical, socioeconomic, and anthropometric characteristics of the children included in the analysis (n = 1,634) did not differ from those who were excluded (n = 1,069, Table S1). The studied children had a median age of 9 years and median F, Pb, Mn, Hg, and Se concentrations were 0.3 μmol/L, 0.5 μg/dL, 10.2 μg/L, 0.3 μg/L, and 178.0 μg/L, respectively. The baseline study population characteristics did not differ by sex (Table 1). Blood metals concentrations were weakly correlated and had a Spearman’s ρ ranging from −0.12 to 0.12 (Figure S2). On the other hand, children’s body size indicators were highly correlated with a Spearman’s ρ >0.9 for BMI and waist circumference (Figure S3).
Table 1.
Selected characteristics of 6-11-year-old children from the 2013-16 NHANES.
| Characteristics | Overall sample (n = 1,634) | Boys (n = 839) | Girls (n = 795) |
|---|---|---|---|
| Age (years) | 9.0 (7.0–10.0)1 | 8.0 (7.0–10.0) | 9.0 (7.0–10.0) |
| BMI2 (kg/m2) | 17.7 (15.7–21.3) | 17.3 (15.6–21.1) | 18.2 (15.8–21.3) |
| Standing height (cm) | 134.3 (125.8–144.2) | 134.3 (126.1–143.4) | 134.5 (125.3–145.0) |
| Waist circumference (cm) | 62.2 (56.3–72.6) | 61.2 (55.9–72.0) | 64.1 (56.9–73.6) |
| Upper arm length (cm) | 28.6 (26.3–32.0) | 28.6 (26.3–0.8) | 28.8 (26.2–31.3) |
| Energy (kcal) | 1,800 (1,406–1,905) | 1,858 (1,480–2350) | 1,753 (1,362–2,168) |
| Outside of school physical activity score | 3.0 (1.0–4.0) | 3.0 (1.0–4.0) | 2.0 (1.0–4.0) |
| At-school physical activity score | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Poverty to income ratio | |||
| ≤ median (1.49) | 855 (52)3 | 431 (51) | 424 (53) |
| > median (1.49) | 779 (48) | 408 (49) | 371 (47) |
| Race | |||
| Mexican American | 571 (35) | 275 (33) | 296 (37) |
| Non-Hispanic white | 450 (28) | 242 (24) | 208 (26) |
| Non-Hispanic black | 398 (24) | 211 (25) | 187 (23) |
| Another race | 215 (13) | 111 (13) | 104 (13) |
| Household members that smoke | 431 (26) | 233 (28) | 198 (25) |
| Cycles | |||
| 2013-14 | 835 (51) | 436 (52) | 399 (50) |
| 2015-16 | 799 (49) | 403 (48) | 396 (50) |
| Metal/trace element concentrations | |||
| F5 | 0.3 (0.1, 0.3–0.5, 4.0)4 | 0.3 (0.1, 0.3–0.5, 3.9) | 0.3 (0.1, 0.2–0.4, 4.0) |
| Pb6 | 0.5 (0.1, 0.4–0.8, 5.8) | 0.5 (0.1, 0.4–0.8, 5.0) | 0.5 (0.1, 0.4–0.7, 5.8) |
| Mn7 | 10.2 (3.8, 8.4–12.6, 24.3) | 9.8 (3.8, 7.9–12.2, 23.4) | 10.7 (4.6, 8.8–13.0, 24.3) |
| Hg7 | 0.3 (0.2, 0.2–0.5, 10.0) | 0.3 (0.2, 0.2–0.5, 7.6) | 0.3 (0.2, 0.2–0.5, 10.0) |
| Se7 | 178.0 (110.2, 167.7–190.2, 238.5) | 177.6 (110.2, 167.1–189.5, 238.5) | 178.5 (138.7, 168.6–190.5, 227.0) |
Continuous variables = median (IQR)
BMI = Body Mass Index
Categorical variables = n (%)
Metal/trace element concentrations = median (min, IQR, max)
Meausred in plasma (μmol/L)
Measured in blood (μg/dL)
Measured in blood (μg/L).
3.2. Bayesian kernel machine regression.
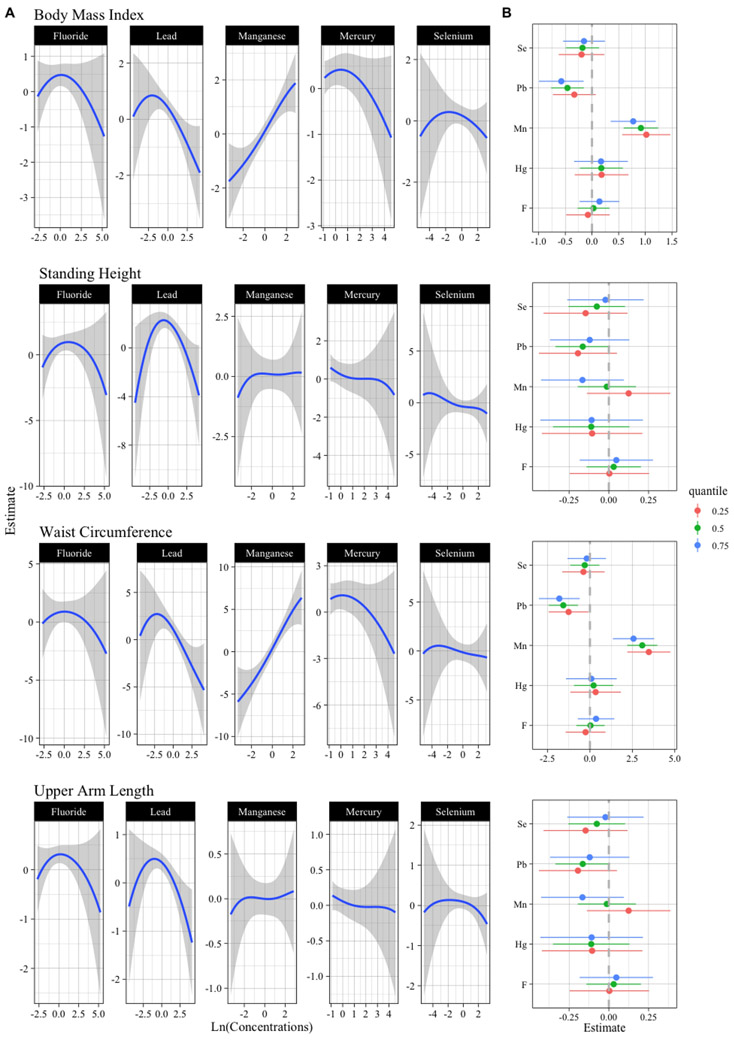
The main findings from the BKMR and linear regression models overall and stratified by sex are summarized in Table S2. Mn was positively associated with children’s BMI and waist circumference (Figure 1A), and this association appeared stronger at lower percentiles of the other metals of the mixture (Figure 1B). Conversely, Pb was negatively associated with BMI and waist circumference (Figure 1A), and this association appeared stronger at higher percentiles of the other metals of the mixture (Figure 1B). These relationships appeared linear; however, there was variability at lower Pb concentrations (Figure 1A). Overall, higher variability at low and high metal concentrations is expected to be related to the limited number of observations at such exposure levels. When we added variable selection in the BKMR models, Mn and Pb were selected for inclusion in more than 50% of iterations (Posterior Inclusion Probability (PIP) >0.5) (Figure S4), and thus they were deemed to be important contributors of the variability of the outcomes (Barbieri and Berger, 2004; Coker et al., 2018; Laue et al., 2020). Effect estimate changes for an IQR increase of each component across the 25th, 50th, and 75th percentiles of the other metals of the mixture suggested Mn and Pb interactions (Figure 1B). The bivariate exposure-response functions support the findings of a Mn-Pb interaction with BMI and waist circumference (Figure S5). Generally, we found similar results in the sex-stratified analyses (Figure S6 and Figure S7); however, with less statistical precision and appearance of nonlinear dose-response, especially among boys. No clear associations were observed with the remaining components of the metal mixture and children’s anthropometric measurements (Figure 1B).
Figure 1:
Metal concentrations and anthropometric indicators of 6-11-year-old children from the 2013-16 NHANES. BKMR dose-response function and interactions within components of the metal mixture (n = 1,634).
Models are adjusted for total calorie intake (kcal, continuous), race (Mexican American, non-Hispanic white, non-Hispanic black or another race, categorical), poverty-to-income ratio (≤1.49 (the median) vs. >1.49), smoker/s in the household (yes vs. no), children’s age (years, continuous), outside-of-school and at-school activity scores (continuous), and child’s sex (boys vs. girls). A) Univariate exposure-response functions and 95% confidence bands for each component with the other components fixed at the median. B) Single component association (estimates and 95% credible intervals, gray dashed line at the null). This plot compares children’ size when a single component is at 75th vs. 25th percentile, when all the other exposures are fixed at either the 25th, 50th, or 75th percentile.
3.3. Multiple metal linear regression.
Using multiple linear regression analyses, an IQR difference in blood Mn was associated with a 0.88 kg/m2 (95% Confidence Interval (CI): 0.59, 1.18) and 2.46 cm (95% CI: 1.69, 3.25) higher BMI and waist circumference, respectively (Table 2). In addition, an IQR change in blood Mn was associated with a 0.20 cm (95% CI: 0.05, 0.35) and 0.70 cm (95% CI: 0.21, 1.19) difference in upper arm length and standing height, respectively (Table 2). The standing height estimate appeared stronger among boys (Table S3). Conversely, higher blood Pb was associated with a lower BMI [−0.47 cm (95% CI: −0.72, −0.21)] and waist circumference [−1.29 cm (95% CI: −1.97, −0.61)], as well as lower upper arm length [−0.24 cm (95% CI: −0.36, −0.11)] and standing height [−0.70 cm (95% CI: −1.13, −0.27)] (Table 2). The positive and negative associations between Mn and Pb concentrations with children’s anthropometry were each consistent in our sex-stratified analyses. We noticed a borderline significant two-way interaction between Mn and Pb with BMI (p-value = 0.057) but attenuated with waist circumference (p-value = 0.133). Further, we observed that an IQR difference in blood Hg concentration was associated with a 0.50 cm (95% CI: −0.97, −0.02) lower standing height (Table 2), particularly among girls [−0.67 (95% CI: −1.34, 0.00)]. Also, among girls, a negative association was found between blood Hg and upper arm length but with wide confidence intervals (Table S3). As in the BKMR analysis, we did not identify associations with other components of the mixture. Additionally, we performed regression models adding metal quantile concentrations (categorical) as independent variable, and the results supported the linear associations previously described (Table S4).
Table 2.
Difference in the BMI, standing height, weight circumference and upper arm length for each IQR difference in metal concentration of 6-11-year-old children from the 2013-16 NHANES.
| Total sample (n = 1,634) |
||||
|---|---|---|---|---|
| BMI (kg/m2) | Standing height (cm) |
Waist circumference (cm) |
Upper arm length (cm) |
|
| Multiple elements β (95% CI)a | ||||
| F | 0.00(−0.24, 0.25) | 0.00(−0.42, 0.41) | 0.10(−0.56, 0.76) | −0.04(−0.16, 0.09) |
| Pb | −0.47(−0.72, −0.21) | −0.70(−1.13, −0.27) | −1.29(−1.97, −0.61) | −0.24(−0.36, −0.11) |
| Mn | 0.88(−0.72, −0.21) | 0.70(−1.13, −0.27) | 2.46(−1.97, −0.61) | 0.20(−0.36, −0.11) |
| Hg | −0.05(−0.33, 0.23) | −0.50(−0.97, −0.02) | −0.20(−0.95, 0.55) | −0.13(−0.27, 0.01) |
| Se | −0.17(−0.44, 0.09) | −0.20(−0.65, 0.25) | −0.31(−1.01, 0.40) | −0.07(−0.20, 0.06) |
Based on linear models adjusted for total calorie intake (kcal, continuous), race (Mexican American, non-Hispanic white, non-Hispanic black or another race, categorical), poverty-to-income ratio (≤1.49 (the median) vs. >1.49), children’s age (years, continuous), smoker/s in the household (yes vs. no), outside-of-school and at-school activity scores (continuous), and children’s sex (boys vs. girls). The models include all five metals (F, Pb, Mn, Hg, and Se).
4. Discussion
Our findings, based on a population of US children with a median age of 9 years participating in the NHANES 2013-16 cycles, suggest that exposure to a metal mixture of F, Mn, Pb, Se, and Hg may influence child body size and growth depending on the exposure levels measured in blood. Plasma F, and whole blood Mn, Pb, Se, and Hg concentrations are considered an accurate/valid biomarker of exposure (Buckley et al., 2020), and the levels found in our population are comparable to other population-based studies (Bose-O’Reilly et al., 2010; Grandjean, 2019; Henn et al., 2010; Hrubá et al., 2012; Kobayashi et al., 2019; Leite et al., 2015; Liu et al., 2014). We observed that Pb concentrations were related to lower BMI and waist circumference at higher concentrations of the other metals of the mixture with little evidence of non-linearity. A negative association was also observed between blood Pb and children’s upper arm length and standing height, especially among boys. Blood Mn, most likely reflecting its function as an essential nutrient, was related to higher BMI and waist circumference at lower concentrations of the other metals, and the shape of the dose-response curve appeared to be linear. A positive association was also observed between Mn concentrations and upper arm length and standing height, especially among boys. Blood Hg concentrations were related to reduced standing height, and this association appeared stronger among girls.
Lead is a known toxic metal. Indeed, no levels of blood Pb are considered safe and childhood exposures have been associated with a broad spectrum of deleterious health effects (Burns et al., 2017). Thus, the CDC reduced the blood Pb reference value from 10 μg/dL to 5 μg/dL in 2012 to minimize risks among vulnerable population groups. Yet, detectable levels of blood Pb among US children persist (Bellinger et al., 2017). In children, high blood Pb concentrations have been associated with lower osteocalcin, a biomarker of bone formation. Pb may interfere with bone cell function, metabolism, and bone mineralization (Mushak et al., 1989; Pounds et al., 1991). For example, Pb may alter circulating levels of hormones required for bone development and maintenance (e.g. 1,25-dihydroxyvitamin D3), as well as the ability of bone cells to respond to hormonal regulation, leading to impaired bone formation. Further, exposure to Pb may have endocrine-disrupting capabilities by reducing responses to hormones that are necessary for growth, such as insulin-like growth factor, and inhibiting the hypothalamic-pituitary-growth axis (Berry et al., 2002; Burns et al., 2017; Deierlein et al., 2019; Fleisch et al., 2013).
Limited epidemiological evidence is available on the effects of low Pb exposure on child growth, particularly as a component of common real-life metal co-exposures. A prior study found that peripubertal blood Pb concentrations in a single measurement were associated with shorter height through age 18 years (Burns et al., 2017). Our study agrees with the prior findings and suggests an inverse association between blood Pb and standing height and upper arm length. In our BKMR models, there was little evidence that Pb interacted with the other components of the metal mixture on these growth measures.
Associations of Pb exposure with indirect estimations of adiposity such as BMI or waist circumference are less commonly reported, and show null (Ballew et al., 1999; Min et al., 2008) and adverse effects (Burns et al., 2017; Cassidy-Bushrow et al., 2016; Deierlein et al., 2019; Little et al., 2009; Schwartz et al., 1986; Scinicariello et al., 2013). Prior studies using NHANES 1976-80 and 1999-2006 data found higher blood Pb associated with lower BMI (Scinicariello et al., 2013) and weight (Schwartz et al., 1986) in children. In contrast, a study using NHANES 1988-94 data did not find associations between blood Pb and children’s weight or BMI (Ballew et al., 1999). In our analysis, a consistent inverse association was observed between Pb exposure and children’s BMI and waist circumference that appeared stronger at higher concentrations of the other metals of the mixture.
Children’s exposure to Mn is a public health concern that points to complexities in establishing exposure thresholds because of its dual role as an essential nutrient required to maintain health, while it is neurotoxic at high levels (Chung et al., 2015; Signes-Pastor et al., 2018). Evidence suggests that Mn follows a U-shaped dose-response curve on children’s neurodevelopment (Henn et al., 2010) and birth size (Chen et al., 2014; Guan et al., 2014; Signes-Pastor et al., 2019; Xia et al., 2016). However, optimal Mn levels have not been defined yet, and there are scant studies on Mn exposure and children’s body size and growth. In this study, we found a linear positive association with children’s body size, with evidence of antagonistic effects of Mn and Pb exposure on children’s BMI and waist circumference. Specifically, the positive association between BMI and waist circumference and blood Mn levels appeared stronger at a lower level of Pb and other mixture components. We found that upper arm length was higher with higher Mn levels, and higher standing height among boys. The Mn exposure levels assessed in our study population appeared to be beneficial for 6-11-year-old children’s growth.
Little is known about the effects of Hg exposure on physical growth. However, it has been suggested that in growing children a mild exposure to Hg associated with a fish-based diet consistent with health recommendations may overcome the known adverse risks of Hg exposure (Benefice et al., 2008; Bose-O’Reilly et al., 2010; Gao et al., 2018; Stratakis et al., 2020). Ingestion of polyunsaturated fatty acids, essential fatty acids with important physiological active functions, are among the main nutritional advantages of a fish-based diet (Benefice et al., 2008; Stratakis et al., 2020). However, there is still controversy regarding the benefits or disadvantages of fish consumption (Mozaffarian and Rimm, 2006; Stratakis et al., 2020). A cross-sectional study from China reported a positive association between blood Hg and children’s anthropometry by comparing 0-6-year-old children from suburban and rural areas with median blood Hg concentrations of 1.34 μg/L and 1.09 μg/L, respectively (Gao et al., 2018). Contrary, we observed that standing height was 0.5 cm lower in children for each IQR difference in Hg concentrations and that upper arm length was about 0.1 cm lower, but the latter association did not reach statistical significance. The Hg effect estimates did not appear to be altered by the other components of the metal mixture. Nevertheless, these findings should be interpreted cautiously given the high proportion of Hg concentrations below the LOD (i.e., 39%). The median blood Hg in our study population was about 4-fold lower compared to the levels reported in the study from China (Gao et al., 2018), which suggests a low seafood consumption. Indeed, low fish and shellfish intake has been previously reported among US children of 6-11 years from NHANES 2013-16 cycles: only 5.8% reported seafood consumption at least twice per week (Terry et al., 2018). Thus, intake of fish and shellfish in our study population is not expected to affect anthropometric indicators, and thus we did not adjust our core models for consumption of marine products. Similarly, sensitivity analysis adjusting linear regression models for marine products including fish and shellfish intake in the past 30 days showed consistent results (data not shown).
Selenium is an essential nutrient, and is a cofactor required by a number of enzymes with antioxidant functions. Its deficiency may lead to modifications in cellular antioxidative capacity and the appearance of a number of diseases. Children are at particular risk for Se deficiency since their rapid growth includes a high demand for Se (Ortega et al., 2013). For instance, lower serum Se levels have been associated with excess weight and reduced height (Lander et al., 2015; Ortega et al., 2013), which supports the epidemiologic data that children need a small amount of Se for normal growth and development (Fábelová et al., 2018). However, our findings provide little evidence of an association between blood Se and children’s anthropometric indicators as no evidence of Se deficiency was observed from their Se blood concentrations. Evidence of Se interactions with the other components of the metal mixture is also limited.
Most of the literature on F exposure is focused on cognitive effects, and suggests a dose-response dependent neurotoxicity at high concentrations but also possibly at even levels below the currently accepted 0.7 mg/L in drinking water for preventive dentistry purposes in the US (Grandjean, 2019). Nonetheless, a study from China investigated the effects of F exposure from drinking water on 6-12-year-old children’s growth, and reported a decreased height among children highly exposed to F at 8.3 mg/L compared to those consuming water at a F level of 0.5 mg/L (Wang et al., 2007). Also, among adults, an increase in bone fractures was associated with drinking water consumption that contained 4 mg/L of F (Institute of Medicine (U.S.), 1997). There is also evidence that F is accumulated in bone and reduces calcium uptake at high F exposure levels, thereby influencing children’s growth and bone strength (Institute of Medicine (U.S.), 1997; Wang et al., 2007). However, in our population of US school children with relatively low plasma F levels, we did not identify any associations between F and anthropometric measurements.
Drinking water is expected to play a major role in metal exposure for our study population in addition to diet and other environmental factors (Buckley et al., 2020; Signes-Pastor et al., 2018). Several metals are naturally occurring in public drinking water sources and others are added for safety and health benefits (e.g., F to prevent cavities), migrated from water pipes (e.g., Pb) or related to industrial activities (Chowdhury et al., 2016; Grandjean, 2019; Ljung et al., 2011). Leafy vegetables, nuts, grains, and animal products are good sources of Mn and Se, while the consumption of certain seafood products is associated with ingestion of Hg (Lucchini et al., 2017; Navarro-Alarcon and Cabrera-Vique, 2008; Stratakis et al., 2020). Nevertheless, metal content in foodstuff can be exacerbated when grown in contaminated soils (Carbonell-Barrachina et al., 2009; Kachenko and Singh, 2006). Contact with chips and dust from toys and household Pb-based paint is of particular concern as a Pb exposure source among children (O’Connor et al., 2018; Shen et al., 2018).
In interpreting our findings, it is important to consider some limitations. First is the cross-sectional nature of the data, where exposures and anthropometric indicators were measured at the same time point. Thus, we cannot draw conclusions regarding temporality of the relationship between metal exposure and children’s body size and growth; these associations will need to be replicated using prospective data with serial measurements in the future. Second, a single blood sample was used to assess metal exposure. Metal concentrations may fluctuate over time, and the use of a single blood sample to assess exposure is based on the assumption that the single measure represents usual exposure levels. Third, our study findings may not be generalizable beyond the age range of 6-11 years because children continue to grow until about 20 years of age, and different toxicants/nutrients may have different critical exposure windows based on the outcomes of interest. In contrast, our study is among the first to flexibly model the association between both nutrient and toxic metals co-exposure at levels relevant to the general population [e.g. median blood Pb was an order of magnitude lower than the reference value of 5 μg/dL (CDC, 2020)] on anthropometric indicators measured in a relatively sizeable sample of US children selected from two NHANES cycles. Our analysis takes advantage of the recently developed nonparametric statistical approach BKMR, which is designed to flexibly assess dose-response, interactions, and mixture effects. BKMR may be poorly suited for very high-dimensional data; however, we only evaluated the exposure to a mixture of 5 metals (Lazarevic et al., 2019). Furthermore, our study explored potential variations by sex given differences in patterns of exposure, gastrointestinal absorption, and/or metabolism (Eriksson et al., 2010; Llop et al., 2013).
In conclusion, our findings suggest that simultaneous exposure to metals may influence children’s body size and growth measurements, and that the effects of metals may differ depending on the exposure levels of other components in the metal mixture. Particularly, our main findings, based on a population of US school-age children, emphasize that further efforts are necessary to reduce Pb exposure from drinking water and other potential sources while maintaining healthy levels of essential nutrients such as Mn to foster growth during the vulnerable period of childhood.
Supplementary Material
Acknowledgments
Funding
This work was funded in part by grants from the National Institutes of Health: P01ES022832, R25CA134286, and P42ES007373, and the US EPA: RD83544201. Gauri Desai was supported by the Community for Global Health Equity at the University at Buffalo. Miguel García-Villarino was funded by CIBERESP (PhD-employment-contract and fellowship for short stays abroad-2019).
Footnotes
Declarations
The authors declare they have no known competing financial interests of personal relationships that could have appeared to influence the work reported in this paper.
References
- Al Osman M, Yang F, Massey IY, 2019. Exposure routes and health effects of heavy metals on children. Biometals Int. J. Role Met. Ions Biol. Biochem. Med 32, 563–573. 10.1007/s10534-019-00193-5 [DOI] [PubMed] [Google Scholar]
- Ballew C, Khan LK, Kaufmann R, Mokdad A, Miller DT, Gunter EW, 1999. Blood lead concentration and children’s anthropometric dimensions in the Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. J. Pediatr 134, 623–630. 10.1016/s0022-3476(99)70250-7 [DOI] [PubMed] [Google Scholar]
- Barbieri MM, Berger JO, 2004. Optimal predictive model selection. Ann. Stat 32, 870–897. 10.1214/009053604000000238 [DOI] [Google Scholar]
- Bellinger DC, Chen A, Lanphear BP, 2017. Establishing and Achieving National Goals for Preventing Lead Toxicity and Exposure in Children. JAMA Pediatr. 171, 616–618. 10.1001/jamapediatrics.2017.0775 [DOI] [PubMed] [Google Scholar]
- Benefice E, Monrroy SJL, Rodriguez RWL, 2008. A nutritional dilemma: fish consumption, mercury exposure and growth of children in Amazonian Bolivia. Int. J. Environ. Health Res 18, 415–427. 10.1080/09603120802272235 [DOI] [PubMed] [Google Scholar]
- Berry WD, Moriarty CM, Lau Y-S, 2002. Lead attenuation of episodic growth hormone secretion in male rats. Int. J. Toxicol 21, 93–98. 10.1080/10915810252866060 [DOI] [PubMed] [Google Scholar]
- Bloom MS, Buck Louis GM, Sundaram R, Maisog JM, Steuerwald AJ, Parsons PJ, 2015. Birth outcomes and background exposures to select elements, the Longitudinal Investigation of Fertility and the Environment (LIFE). Environ. Res 138, 118–129. 10.1016/j.envres.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Claus Henn B, Valeri L, Coull BA, 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health Glob. Access Sci. Source 17, 1–10. 10.1186/s12940-018-0413-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA, 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostat. Oxf. Engl 16, 493–508. 10.1093/biostatistics/kxu058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose-O’Reilly S, McCarty KM, Steckling N, Lettmeier B, 2010. Mercury Exposure and Children’s Health. Curr. Probl. Pediatr. Adolesc. Health Care 40, 186–215. 10.1016/j.cppeds.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Barrett ES, Beamer PI, Bennett DH, Bloom MS, Fennell TR, Fry RC, Funk WE, Hamra GB, Hecht SS, Kannan K, Iyer R, Karagas MR, Lyall K, Parsons PJ, Pellizzari ED, Signes-Pastor AJ, Starling AP, Wang A, Watkins DJ, Zhang M, Woodruff TJ, 2020. Opportunities for evaluating chemical exposures and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) Program. J. Expo. Sci. Environ. Epidemiol 1–23. 10.1038/s41370-020-0211-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JS, Williams PL, Lee MM, Revich B, Sergeyev O, Hauser R, Korrick SA, 2017. Peripubertal blood lead levels and growth among Russian boys. Environ. Int 106, 53–59. 10.1016/j.envint.2017.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell-Barrachina AA, Signes-Pastor AJ, Vázquez-Araújo L, Burló F, Sengupta B, 2009. Presence of arsenic in agricultural products from arsenic-endemic areas and strategies to reduce arsenic intake in rural villages. Mol. Nutr. Food Res 53, 531–541. 10.1002/mnfr.200900038 [DOI] [PubMed] [Google Scholar]
- Cassidy-Bushrow AE, Havstad S, Basu N, Ownby David R., Park SK, Ownby Dennis R., Johnson CC, Wegienka G, 2016. Detectable Blood Lead Level and Body Size in Early Childhood. Biol. Trace Elem. Res 171, 41–47. 10.1007/s12011-015-0500-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2020. Lead - Blood Lead Reference Value. https://www.cdc.gov/nceh/lead/data/blood-lead-reference-value.htm (accessed 4.1.20).
- Chen L, Ding G, Gao Y, Wang P, Shi R, Huang H, Tian Y, 2014. Manganese concentrations in maternal–infant blood and birth weight. Environ. Sci. Pollut. Res 21, 6170–6175. 10.1007/s11356-013-2465-4 [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Mazumder MAJ, Al-Attas O, Husain T, 2016. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ 569–570, 476–488. 10.1016/j.scitotenv.2016.06.166 [DOI] [PubMed] [Google Scholar]
- Chung SE, Cheong H-K, Ha E-H, Kim B-N, Ha M, Kim Y, Hong Y-C, Park H, Oh S-Y, 2015. Maternal Blood Manganese and Early Neurodevelopment: The Mothers and Children’s Environmental Health (MOCEH) Study. Environ. Health Perspect 123, 717–722. 10.1289/ehp.1307865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker E, Chevrier J, Rauch S, Bradman A, Obida M, Crause M, Bornman R, Eskenazi B, 2018. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environ. Int 113, 122–132. 10.1016/j.envint.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks DL, 1995. American children at risk: Poverty and its consequences for children’s health, growth, and school achievement. Am. J. Phys. Anthropol 38, 57–86. 10.1002/ajpa.1330380605 [DOI] [Google Scholar]
- Deierlein AL, Teitelbaum SL, Windham GC, Pinney SM, Galvez MP, Caldwell KL, Jarrett JM, Gajek R, Kushi LH, Biro F, Wolff MS, Breast Cancer and Environment Research Program, 2019. Lead exposure during childhood and subsequent anthropometry through adolescence in girls. Environ. Int 122, 310–315. 10.1016/j.envint.2018.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJP, 2010. Boys live dangerously in the womb. Am. J. Hum. Biol. Off. J. Hum. Biol. Counc 22, 330–335. 10.1002/ajhb.20995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fábelová L, Vandentorren S, Vuillermoz C, Garnier R, Lioret S, Botton J, 2018. Hair concentration of trace elements and growth in homeless children aged <6years: Results from the ENFAMS study. Environ. Int 114, 318–325. 10.1016/j.envint.2017.10.012 [DOI] [PubMed] [Google Scholar]
- Fleisch AF, Burns JS, Williams PL, Lee MM, Sergeyev O, Korrick SA, Hauser R, 2013. Blood Lead Levels and Serum Insulin-Like Growth Factor 1 Concentrations in Peripubertal Boys. Environ. Health Perspect 121, 854–858. 10.1289/ehp.1206105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Amaya E, Gil F, Murcia M, Llop S, Casas M, Vrijheid M, Lertxundi A, Irizar A, Fernández-Tardón G, Castro-Delgado RV, Olea N, Fernández MF, 2019. Placental metal concentrations and birth outcomes: The Environment and Childhood (INMA) project. Int J Hyg Environ Health 222, 468–478. 10.1016/j.ijheh.2018.12.014 [DOI] [PubMed] [Google Scholar]
- Gao Z-Y, Li M-M, Wang J, Yan J, Zhou C-C, Yan C-H, 2018. Blood mercury concentration, fish consumption and anthropometry in Chinese children: A national study. Environ. Int 110, 14–21. 10.1016/j.envint.2017.08.016 [DOI] [PubMed] [Google Scholar]
- Gardner RM, Kippler M, Tofail F, Bottai M, Hamadani J, Grandér M, Nermell B, Palm B, Rasmussen KM, Vahter M, 2013. Environmental Exposure to Metals and Children’s Growth to Age 5 Years: A Prospective Cohort Study. Am. J. Epidemiol 177, 1356–1367. 10.1093/aje/kws437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelander L, 2006. Children’s growth: A health indicator and a diagnostic tool. Acta Paediatr. 95, 517–518. 10.1111/j.1651-2227.2006.tb02276.x [DOI] [PubMed] [Google Scholar]
- Gleason KM, Valeri L, Shankar AH, Hasan MOSI, Quamruzzaman Q, Rodrigues EG, Christiani DC, Wright RO, Bellinger DC, Mazumdar M, 2016. Stunting is associated with blood lead concentration among Bangladeshi children aged 2-3 years. Environ. Health 15, 103. 10.1186/s12940-016-0190-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govarts E, Remy S, Bruckers L, Den Hond E, Sioen I, Nelen V, Baeyens W, Nawrot TS, Loots I, Van Larebeke N, Schoeters G, 2016. Combined Effects of Prenatal Exposures to Environmental Chemicals on Birth Weight. Int. J. Environ. Res. Public. Health 13. 10.3390/ijerph13050495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, 2019. Developmental fluoride neurotoxicity: an updated review. Environ. Health 18, 110. 10.1186/s12940-019-0551-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Wang M, Li X, Piao F, Li Q, Xu L, Kitamura F, Yokoyama K, 2014. Manganese concentrations in maternal and umbilical cord blood: related to birth size and environmental factors. Eur. J. Public Health 24, 150–157. 10.1093/eurpub/ckt033 [DOI] [PubMed] [Google Scholar]
- Henn BC, Ettinger AS, Schwartz J, Téllez-Rojo MM, Lamadrid-Figueroa H, Hernández-Avila M, Schnaas L, Amarasiriwardena C, Bellinger DC, Hu H, Wright RO, 2010. Early Postnatal Blood Manganese Levels and Children’s Neurodevelopment. Epidemiol. Camb. Mass 21, 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrubá F, Strömberg U, Černá M, Chen C, Harari F, Harari R, Horvat M, Koppová K, Kos A, Krsková A, Krsnik M, Laamech J, Li Y-F, Löfmark L, Lundh T, Lundström N-G, Lyoussi B, Mazej D, Osredkar J, Pawlas K, Pawlas N, Prokopowicz A, Rentschler G, Spěváčková V, Spiric Z, Tratnik J, Skerfving S, Bergdahl IA, 2012. Blood cadmium, mercury, and lead in children: An international comparison of cities in six European countries, and China, Ecuador, and Morocco. Environ. Int 41, 29–34. 10.1016/j.envint.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (U.S.) (Ed.), 1997. Dietary reference intakes: for calcium, phosphorus, magnesium, vitamin D, and fluoride. National Academy Press, Washington, D.C. [PubMed] [Google Scholar]
- Kachenko AG, Singh B, 2006. Heavy Metals Contamination in Vegetables Grown in Urban and Metal Smelter Contaminated Sites in Australia. Water. Air. Soil Pollut 169, 101–123. 10.1007/s11270-006-2027-1 [DOI] [Google Scholar]
- Kerr BT, Ochs-Balcom HM, López P, García-Vargas GG, Rosado JL, Cebrián ME, Kordas K, 2019. Effects of ALAD genotype on the relationship between lead exposure and anthropometry in a Cohort of Mexican children. Environ. Res 170, 65–72. 10.1016/j.envres.2018.12.003 [DOI] [PubMed] [Google Scholar]
- Kim Sujin, Kim Sunmi, Won S., Choi K., 2017. Considering common sources of exposure in association studies - Urinary benzophenone-3 and DEHP metabolites are associated with altered thyroid hormone balance in the NHANES 2007-2008. Environ. Int 107, 25–32. 10.1016/j.envint.2017.06.013 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Kishi R, Saijo Y, Ito Y, Oba K, Araki A, Miyashita C, Itoh S, Minatoya M, Yamazaki K, Ait Bamai Y, Sato T, Yamazaki S, Nakayama SF, Isobe T, Nitta H, Japan Environment and Children’s Study Group, 2019. Association of blood mercury levels during pregnancy with infant birth size by blood selenium levels in the Japan Environment and Children’s Study: A prospective birth cohort. Environ. Int 125, 418–429. 10.1016/j.envint.2019.01.051 [DOI] [PubMed] [Google Scholar]
- Lander RL, Williams SM, Costa-Ribeiro H, Mattos AP, Barreto DL, Houghton LA, Bailey KB, Lander AG, Gibson RS, 2015. Understanding the complex determinants of height and adiposity in disadvantaged daycare preschoolers in Salvador, NE Brazil through structural equation modelling. BMC Public Health 15, 1086. 10.1186/s12889-015-2406-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue HE, Moroishi Y, Jackson BP, Palys TJ, Madan JC, Karagas MR, 2020. Nutrient-toxic element mixtures and the early postnatal gut microbiome in a United States longitudinal birth cohort. Environ. Int 138, 105613. 10.1016/j.envint.2020.105613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic N, Barnett AG, Sly PD, Knibbs LD, 2019. Statistical Methodology in Studies of Prenatal Exposure to Mixtures of Endocrine-Disrupting Chemicals: A Review of Existing Approaches and New Alternatives. Environ. Health Perspect 127, 1–24. 10.1289/EHP2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite HP, Nogueira PCK, de O Iglesias SB., de Oliveira SV, Sarni ROS, 2015. Increased plasma selenium is associated with better outcomes in children with systemic inflammation. Nutr. Burbank Los Angel. Cty. Calif 31, 485–490. 10.1016/j.nut.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Lewicka I, Kocyłowski R, Grzesiak M, Gaj Z, Oszukowski P, Suliburska J, 2017. Selected trace elements concentrations in pregnancy and their possible role - literature review. Ginekol. Pol 88, 509–514. 10.5603/GP.a2017.0093 [DOI] [PubMed] [Google Scholar]
- Little BB, Spalding S, Walsh B, Keyes DC, Wainer J, Pickens S, Royster M, Villanacci J, Gratton T, 2009. Blood lead levels and growth status among African–American and Hispanic children in Dallas, Texas – 1980 and 2002: Dallas Lead Project II. Ann. Hum. Biol 36, 331–341. 10.1080/03014460902806615 [DOI] [PubMed] [Google Scholar]
- Liu J, Liu X, Wang W, McCauley L, Pinto-Martin J, Wang Y, Li L, Yan C, Rogan WJ, 2014. Blood Lead Concentrations and Children’s Behavioral and Emotional Problems: A Cohort Study. JAMA Pediatr. 168, 737–745. 10.1001/jamapediatrics.2014.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Palm B, Grandér M, Vahter M, 2011. High concentrations of essential and toxic elements in infant formula and infant foods - A matter of concern. Food Chem. 127, 943–951. 10.1016/j.foodchem.2011.01.062 [DOI] [PubMed] [Google Scholar]
- Llop S, Lopez-Espinosa M-J, Rebagliato M, Ballester F, 2013. Gender differences in the neurotoxicity of metals in children. Toxicology 311, 3–12. 10.1016/j.tox.2013.04.015 [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P, 2004. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environ. Health Perspect 112, 1691–1696. 10.1289/ehp.7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini R, Placidi D, Cagna G, Fedrighi C, Oppini M, Peli M, Zoni S, 2017. Manganese and Developmental Neurotoxicity. Adv. Neurobiol 18, 13–34. 10.1007/978-3-319-60189-2_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K-B, Min J-Y, Cho S-I, Kim R, Kim H, Paek D, 2008. Relationship between low blood lead levels and growth in children of white-collar civil servants in Korea. Int. J. Hyg. Environ. Health 211, 82–87. 10.1016/j.ijheh.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Rimm EB, 2006. Fish Intake, Contaminants, and Human Health: Evaluating the Risks and the Benefits. JAMA 296, 1885–1899. 10.1001/jama.296.15.1885 [DOI] [PubMed] [Google Scholar]
- Mushak P, Michael Davis J, Crocetti AF, Grant LD, 1989. Prenatal and postnatal effects of low-level lead exposure: Integrated summary of a report to the U.S. congress on childhood lead poisoning. Environ. Res 50, 11–36. 10.1016/S0013-9351(89)80046-5 [DOI] [PubMed] [Google Scholar]
- Navarro-Alarcon M, Cabrera-Vique C, 2008. Selenium in food and the human body: A review. Sci. Total Environ 400, 115–141. 10.1016/j.scitotenv.2008.06.024 [DOI] [PubMed] [Google Scholar]
- NHANES, 2018. 2015-2016 Data Documentation, Codebook, and Frequencies, Centers for Disease Control and Prevention. https://doi.org/wwwn.cdc.gov/Nchs/Nhanes/2015-2016/PBCD_I.htm [Google Scholar]
- NHANES, 2016a. NHANES 2015-2016 Anthropometry Procedures Manual, Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_Anthropometry_Procedures_Manual.pdf [Google Scholar]
- NHANES, 2016b. 2013-2014 Data Documentation, Codebook, and Frequencies. Cent. Dis. Control Prev. [Google Scholar]
- NHANES, 2015a. NHANES 2015-2016 Laboratory Methods, Centers for Disease Control and Prevention. https://doi.org/wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2015 [Google Scholar]
- NHANES, 2015b. NHANES 2015-2016 Procedure Manuals, Centers for Disease Control and Prevention. https://doi.org/wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Manuals.aspx?BeginYear=2015 [Google Scholar]
- NHANES, 2013a. NHANES 2013-2014 Laboratory Methods, Centers for Disease Control and Prevention. https://doi.org/wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/LabMethods.aspx?BeginYear=2013 [Google Scholar]
- NHANES, 2013b. NHANES 2013-2014 Procedure Manuals, Centers for Disease Control and Prevention. https://doi.org/wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Manuals.aspx?BeginYear=2013 [Google Scholar]
- NHANES, 2013c. Anthropometry Procedures Manual 2013, Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013_Anthropometry.pdf [Google Scholar]
- O’Connor D, Hou D, Ye J, Zhang Y, Ok YS, Song Y, Coulon F, Peng T, Tian L, 2018. Lead-based paint remains a major public health concern: A critical review of global production, trade, use, exposure, health risk, and implications. Environ. Int 121, 85–101. 10.1016/j.envint.2018.08.052 [DOI] [PubMed] [Google Scholar]
- Ortega M, Rodríguez-Rodríguez Aparicio, Jiménez-Ortega I, Palmeros Perea, Navia M, López-Sobaler M, 2013. Young Children with Excess of Weight Show an Impaired Selenium Status. Int. J. Vitam. Nutr. Res [DOI] [PubMed] [Google Scholar]
- Pounds JG, Long GJ, Rosent JF, 1991. Cellular and molecular toxicity of lead in bone. 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2014. R: A Language and Enrionment for Statistical Computing, R Foundation for Statisical Computing. Vienna. https://doi.org/www.r-project.org/ [Google Scholar]
- Raihan MJ, Briskin E, Mahfuz M, Islam MM, Mondal D, Hossain MI, Ahmed AMS, Haque R, Ahmed T, 2018. Examining the relationship between blood lead level and stunting, wasting and underweight- A cross-sectional study of children under 2 years-of-age in a Bangladeshi slum. PloS One 13, e0197856. 10.1371/journal.pone.0197856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman MP, 2012. Selenium and human health. The Lancet 379, 1256–1268. 10.1016/S0140-6736(11)61452-9 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, Alguacil J, Gil F, González-Alzaga B, Rojas-García A, 2013. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: A systematic review and meta-analysis. Sci. Total Environ 454–455, 562–577. 10.1016/j.scitotenv.2013.03.047 [DOI] [PubMed] [Google Scholar]
- Sabra S, Malmqvist E, Saborit A, Gratacós E, Gomez Roig MD, 2017. Heavy metals exposure levels and their correlation with different clinical forms of fetal growth restriction. PloS One 12, e0185645. 10.1371/journal.pone.0185645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Vexler A, Whitcomb BW, Liu A, 2006. The Limitations due to Exposure Detection Limits for Regression Models. Am. J. Epidemiol 163, 374–383. 10.1093/aje/kwj039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Angle C, Pitcher H, 1986. Relationship between childhood blood lead levels and stature. Pediatrics 77, 281–288. [PubMed] [Google Scholar]
- Scinicariello F, Buser MC, Mevissen M, Portier CJ, 2013. Blood lead level association with lower body weight in NHANES 1999-2006. Toxicol. Appl. Pharmacol 273, 516–523. 10.1016/j.taap.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Hou D, Zhang P, Wang Y, Zhang Y, Shi P, O’Connor D, 2018. Lead-based paint in children’s toys sold on China’s major online shopping platforms. Environ. Pollut 241, 311–318. 10.1016/j.envpol.2018.05.078 [DOI] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Bouchard MF, Baker E, Jackson BP, Karagas MR, 2018. Toenail manganese as biomarker of drinking water exposure: a reliability study from a US pregnancy cohort. J. Expo. Sci. Environ. Epidemiol 1. 10.1038/s41370-018-0108-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Doherty BT, Romano ME, Gleason KM, Gui J, Baker E, Karagas MR, 2019. Prenatal exposure to metal mixture and sex-specific birth outcomes in the New Hampshire Birth Cohort Study. Environ. Epidemiol 10.1097/EE9.0000000000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratakis N, Conti DV, Borras E, Sabido E, Roumeliotaki T, Papadopoulou E, Agier L, Basagana X, Bustamante M, Casas M, Farzan SF, Fossati S, Gonzalez JR, Grazuleviciene R, Heude B, Maitre L, McEachan RRC, Theologidis I, Urquiza J, Vafeiadi M, West J, Wright J, McConnell R, Brantsaeter A-L, Meltzer H-M, Vrijheid M, Chatzi L, 2020. Association of Fish Consumption and Mercury Exposure During Pregnancy With Metabolic Health and Inflammatory Biomarkers in Children. JAMA Netw. Open 3, e201007–e201007. 10.1001/jamanetworkopen.2020.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry A, Herrick K, Afful J, Ahluwalia N, 2018. Seafood Consumption in the United States, 2013–2016 [WWW Document]. URL https://www.cdc.gov/nchs/products/databriefs/db321.htm (accessed 4.2.20). [PubMed]
- Thomas S, Arbuckle TE, Fisher M, Fraser WD, Ettinger A, King W, 2015. Metals exposure and risk of small-for-gestational age birth in a Canadian birth cohort: The MIREC study. Environ. Res 140, 430–439. 10.1016/j.envres.2015.04.018 [DOI] [PubMed] [Google Scholar]
- Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, Kile ML, Quamruzzaman Q, Afroz S, Golam M, Amarasiriwardena C, Bellinger DC, Christiani DC, Coull BA, Wright RO, 2017. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20-40 Months of Age: Evidence from Rural Bangladesh. Environ. Health Perspect 067015, 1–11. 10.1289/EHP614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-X, Wang Z-H, Cheng X-T, Li J, Sang Z-P, Zhang X-D, Han L-L, Qiao X-Y, Wu Z-M, Wang Z-Q, 2007. Arsenic and fluoride exposure in drinking water: children’s IQ and growth in Shanyin county, Shanxi province, China. Environ. Health Perspect 115, 643–647. 10.1289/ehp.9270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gao X, Pedram P, Shahidi M, Du J, Yi Y, Gulliver W, Zhang H, Sun G, 2016. Significant Beneficial Association of High Dietary Selenium Intake with Reduced Body Fat in the CODING Study. Nutrients 8. 10.3390/nu8010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Zhou Y, Zheng T, Zhang B, Bassig BA, Li Y, Wise JP, Zhou A, Wan Y, Wang Y, Xiong C, Zhao J, Li Z, Yao Y, Hu J, Pan X, Xu S, 2016. Maternal urinary manganese and risk of low birth weight: a case–control study. BMC Public Health 16, 1–9. 10.1186/s12889-016-2816-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Huo X, Yekeen TA, Zheng Q, Zheng M, Xu X, 2013. Effects of lead and cadmium exposure from electronic waste on child physical growth. Environ. Sci. Pollut. Res 20, 4441–4447. 10.1007/s11356-012-1366-2 [DOI] [PubMed] [Google Scholar]
- Zeng X, Xu X, Qin Q, Ye K, Wu W, Huo X, 2019. Heavy metal exposure has adverse effects on the growth and development of preschool children. Environ. Geochem. Health 41, 309–321. 10.1007/s10653-018-0114-z [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dong T, Hu W, Wang Xu, Xu B, Lin Z, Hofer T, Stefanoff P, Chen Y, Wang Xinru, Xia Y, 2019. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int 123, 325–336. 10.1016/j.envint.2018.11.076 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.