Abstract
Chronic active Epstein-Barr virus infection is a rare disease with an often fatal outcome. Cardiovascular complications are associated with a poor prognosis. We herein describe the clinical course of an adult patient with Epstein-Barr virus-associated systemic vasculitis complicated by multi-systemic aneurysmal disease. The vascular imaging showed multiple aneurysms involving coronary arteries, abdominal arteries, cerebral arteries, and vertebral arteries. Immunophenotyping analysis of peripheral blood lymphocytes revealed the presence of an increased number of double negative T cells. The patient received multiple lines of immunosuppressive therapy with no response. Unfortunately, he succumbed to a cerebral aneurysm rupture.
Introduction
Epstein-Barr virus (EBV) is a ubiquitous virus which infects over 90% of the world population and persists for a lifetime. Whereas most EBV infections of children are asymptomatic or have nonspecific symptoms, infections of adolescents and adults frequently result in infectious mononucleosis (IM). Chronic Active Epstein-Barr Virus Infection (CAEBV) is a very rare disorder characterized by chronic or recurrent IM-like symptoms and an unusual pattern of anti EBV antibodies. The pathogenesis of this infection is still unclear and is suggested to have a correlation with clonal proliferation of EBV infected T or NK cells and their infiltration into systemic organs leading to their failure.1 CAEBV is a progressive bi-faceted disease with inflammatory and neoplastic elements, associated with high mortality and morbidity. Life threatening complications could develop during the disease course such as hemophagocytic lymphohistiocytosis (HLH), lymphoma, interstitial pneumonia, cardiovasculopathy and central nervous system (CNS) involvement. Coronary artery aneurysms and myocarditis are the major cardiovascular complications correlated with poor prognosis of CAEBV. Most of reported cases are pediatric patients. We herein report an exceptional case of systemic vasculitis with multiple aneurysms complicating CAEBV in an adult patient.
Case Presentation
A previously healthy 22-year-old Moroccan male was admitted to our hospital in August 2016 with a three-month history of fever of unknown origin. The patient was complaining of headache, neck pain, diffuse myalgia, and night sweats. Physical examination on admission found a mild bilateral conjunctival injection, a bilateral periorbital edema, a labial edema, cervical lymphadenopathy, and hepatosplenomegaly. Cardiac auscultation noted normal heart sounds and no murmurs. Neurological examination didn’t reveal any abnormalities. Blood count at presentation showed pancytopenia: hemoglobin 11 g/dL, white blood cells 3400/μL, and platelets 103000/μL. Blood biochemistry revealed increased levels of C-reactive protein (33 mg/L), ferritin (2773 ng/mL), triglycerides (189 mg/dL), lactate dehydrogenase (1179 IU/L), and liver enzymes (aspartate aminotransferase 587 IU/L, alanine aminotransferase 428 IU/L). The level of fibrinogen was normal. Prothrombin time and activated partial thromboplastin time were within the normal levels. Bone marrow aspiration showed active hemophagocytosis (Figure 1), 9% of dystrophic plasma cells, and 12% of promyelocytes containing toxic granulations. The osteomedullary biopsy showed a discretely hypoplastic bone marrow with no lymphomatous infiltration and no histological features of malignancy. Repeated aerobic and anaerobic blood cultures were persistently negative. Echocardiography didn’t show any sign of infective endocarditis. Chest radiography didn’t show any abnormalities. Screening for tuberculosis infection was negative (interferon-gamma release assay, sputum culture, and GeneXpert). Hepatitis B and C, human immunodeficiency virus (HIV), cytomegalovirus (CMV), and parvovirus B19 serologic tests were all negative. The specific serologic tests for EBV were all positive: EBV-VCA-IgM, EBV-VCA-IgG, EBV-EA-IgG, and EBV-EBNA-IgG. Real-time polymerase chain reaction (RT-PCR) detected a high EBV load in the peripheral blood (164000 IU/mL, 5.21 Log IU/mL). The histopathological examination of a liver biopsy showed a diffuse lymphocytic infiltrate with sinusoidal dilatation, and in-situ hybridization test was positive for EBV-encoded small RNA (EBER). A lymph node biopsy showed non-neoplastic lymphoid hyperplasia. Peripheral blood lymphocyte immunophenotyping by flow cytometry revealed a decreased population of natural killer cells (24/mm3 [70–400]) and an increased population of double negative T cells CD3+ CD4− CD8− (670/mm3 [<150/mm3]). The immunological assessment didn’t show any autoimmune pathology. Antinuclear antibodies, extractable nuclear antigen antibodies, rheumatoid factor, anti-neutrophil cytoplasmic antibodies, and cryoglobulinemia were all negative. There was no evidence of congenital or acquired immunodeficiency diseases: no family history of a primary immunodeficiency, no personal history of recurrent infections or growth delay, no neutropenia or lymphopenia; quantitative immunoglobulin (Ig) measurements and complement components assessment (CH50, C3, C4) were normal. Based on these findings, the patient was diagnosed with HLH-complicated CAEBV and was treated with dexamethasone (10 mg/m2 daily for two weeks, then 5 mg/m2 daily for two weeks, then 2.5 mg/m2 daily for two weeks, then 1.25 mg/m2 daily for two weeks), cyclosporin A (6 mg/kg daily for eight weeks), and etoposide (150 mg/m2 twice a week for two weeks, then once a week for six weeks). The patient was discharged from the hospital in January 2017. Chemotherapy was continued every second week for six months. Despite these intensive treatments, fever and splenomegaly persisted. Follow up evaluation showed a moderate decrease in the ferritin level and a normalization of the triglyceride level, but a worsening of thrombocytopenia.
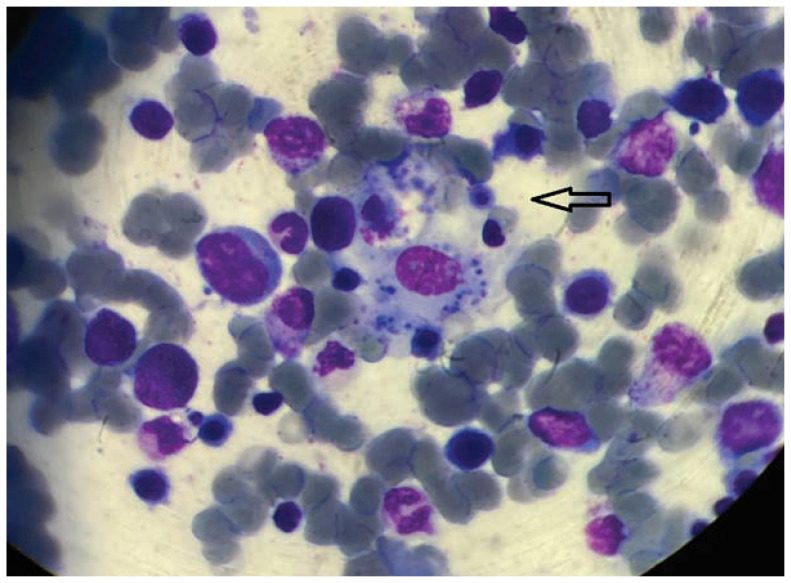
Figure 1.
Hemophagocytosis Bone marrow aspirate smear showing features of hemophagocytosis.
The patient was readmitted to the hospital in November 2017 following the deterioration of his condition. He complained of dyspnea, chest pain, abdominal pain, and generalized asthenia. Contrast-enhanced Computed Tomography Scan (CT-Scan) showed multiple aneurysms involving coronary arteries (Figure 2A) and abdominal arteries (Figure 3). Transthoracic echocardiography showed giant aneurysms involving the left main coronary artery (Figure 2C) and the left anterior descending coronary artery. Additionally, coronary angiography revealed multiple aneurysms involving the circumflex artery and the right coronary artery (Figure 2B). Abdominal angiography revealed multiple aneurysms involving the hepatic artery, the gastroduodenal artery, the splenic artery, the superior mesenteric artery, as well as the common iliac arteries. The patient was diagnosed with CAEBV-associated systemic vasculitis and was administered ganciclovir (5 mg/kg twice a day for three weeks), corticosteroids (3 pulses of methylprednisolone, 1,000 mg each; followed by prednisone at a dose of 1 mg/kg/day with gradual tapering), intravenous immunoglobulins (0.4 g/kg/day for five days), and intravenous cyclophosphamide (15 mg/kg every two weeks for a month, then every three weeks for three months) without significant benefits. It was, therefore, decided to start bortezomib therapy (1.3 mg/m2 twice a week for two weeks, followed by a 10-day rest period), which was discontinued after two cycles because of severe thrombocytopenia. The patient was discharged from the hospital in April 2018. Systemic corticosteroid therapy was continued on an outpatient basis.
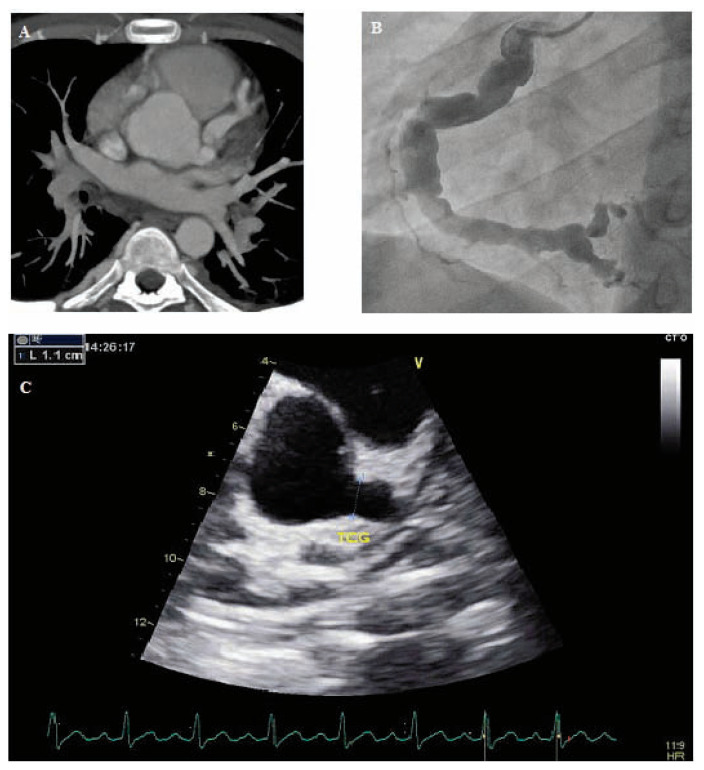
Figure 2.
Coronary artery aneurysms
Panel A. Cross-sectional image of a CT-scan showing an aneurysmal dilation of the left main coronary artery and the left anterior descending artery.
Panel B. Coronary angiography showing an ectasia on the right coronary artery with multiple aneurysms.
Panel C. Echocardiography showing a giant aneurysm involving the left main coronary artery (11 mm in diameter).
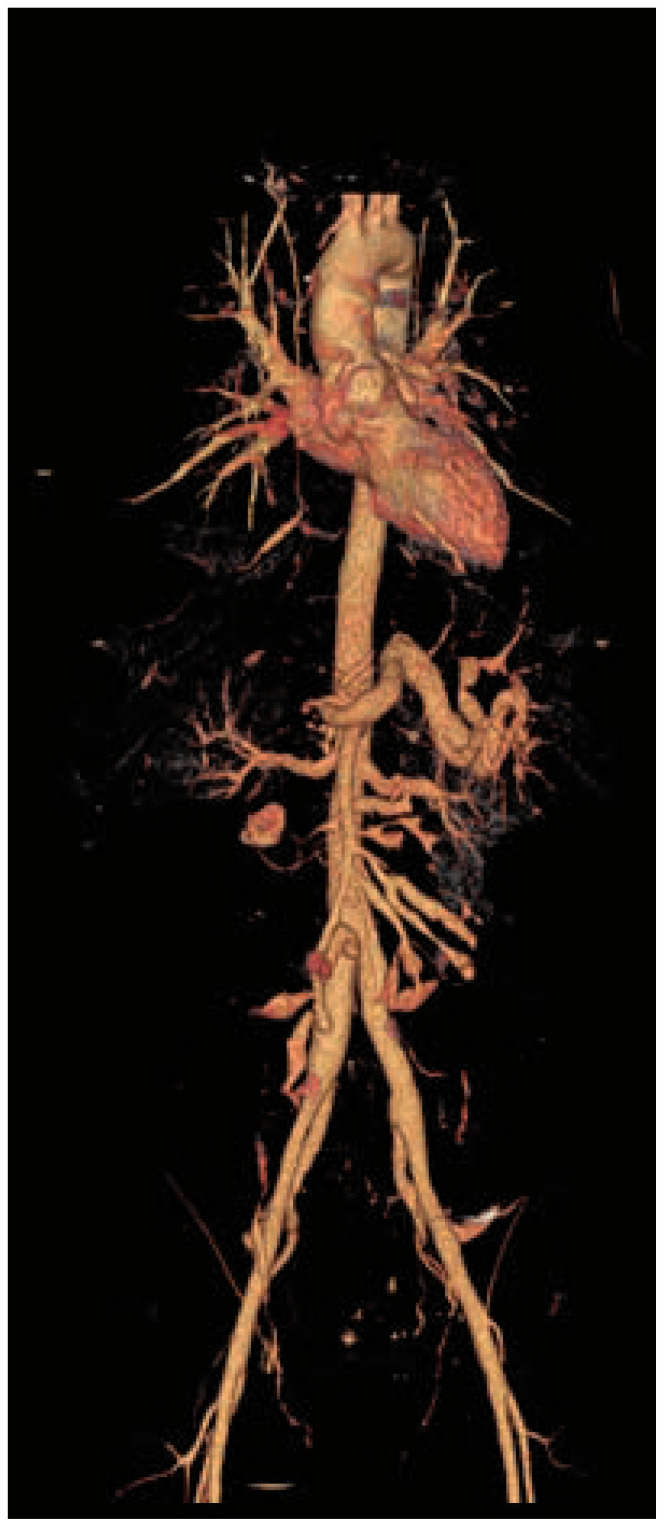
Figure 3.
Abdominal artery aneurysms
Volume Rendering Technique (VRT) reconstruction of a CT-scan showing an aneurysmal dilation of the gastroduodenal artery, the splenic artery, and the branches of the superior mesenteric artery.
In July 2018, the patient presented with fever, abdominal pain, and bloody diarrhea. Abdominal CT-Scan showed an increase in number and size of arterial aneurysms. Endoscopic exploration showed the presence of two linear ulcerations in the left colon. The histopathological examination of colonic biopsy specimens showed basally located lymphoid aggregates, and an in-situ hybridization test was positive for EBER. The patient received GEMOX chemotherapy regimen (Gemcitabine 1,000 mg/m2 and Oxaliplatin 100 mg/m2, every 14 days) which was discontinued after one cycle because of acute hepatic cytolysis.
One month later, the patient was admitted to the Intensive Care Unit with altered mental status. Brain CT-Scan revealed a large temporal lobe hematoma. He then developed status epilepticus, and this was successfully treated with clonazepam and levetiracetam. Brain magnetic resonance angiography revealed multiple micro-aneurysms of the anterior and posterior cerebral arteries, as well as bilateral vertebral artery aneurysms (Figure 4). His level of consciousness improved after 21 days and he was transferred to the Department of Onco-Hematology where it was decided to perform an allogeneic hematopoietic stem cell transplantation (HSCT). Unfortunately, the patient died 10 days after the start of the conditioning regimen, following a second cerebral aneurysm rupture in November 2018.
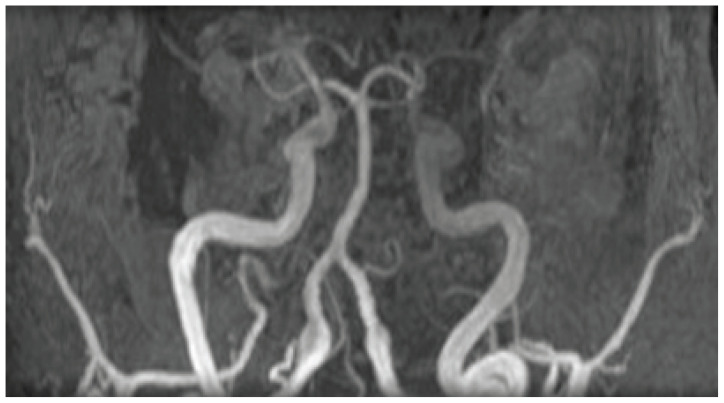
Figure 4.
Vertebral artery aneurysms. 3D reconstruction of a cerebral Magnetic Resonance Angiography (MRA) showing a fusiform dilation of the vertebral arteries.
Discussion
We herein report an exceptional case of systemic vasculitis associated with CAEBV in an adult patient. To the best of our knowledge, this is the first reported case of multi-systemic aneurysmal disease in an adult patient fulfilling the diagnostic criteria for CAEBV.
CAEBV was originally reported as a “chronic infectious mononucleosis”2 or “atypical severe illness associated with serological evidence of persistent EBV infection.”3 Most cases were reported in Japan and East Asia. In 2005, Okano et al. proposed the first diagnostic guidelines as follows:4 1) persistent or recurrent IM-like symptoms, generally including fever, swelling of lymph nodes, and hepatosplenomegaly; 2) unusual pattern of anti EBV antibodies with raised anti VCA (VCA-IgG ≥ 1:640) and anti EA (EA-IgG ≥ 1:160) and/or detection of increased EBV genomes in affected tissues, including the peripheral blood; 3) chronic illness which cannot be explained by other known disease processes at the time of diagnosis (autoimmune diseases, immunodeficiency diseases, malignancies). In the revised World Health Organization classification of tumors of hematopoietic and lymphoid tissues (2016), CAEBV was classified in the category of EBV-positive T or NK cell lymphoproliferative diseases.5
Distinguishing CAEBV from other EBV-positive T and NK cell proliferations can be challenging. Persons with congenital or acquired immunodeficiencies can develop severe lymphoproliferative disorders associated with primary EBV infection or reactivation of the virus. In fact, impairment in T cell proliferation, B cell – T cell interactions, and T cell and NK cell cytotoxicity can result in failure to adequately control EBV infection. Some authors reported specific immune deficiencies associated with severe EBV disease: X-linked lymphoproliferative disease 1 (XLP1) due to a mutation in SAP/SH2D1A gene, X-linked lymphoproliferative disease 2 (XLP2) due to a mutation in BIRC4, IL-2 inducible T cell kinase deficiency, CD27/CD70 deficiency, and mutations in Magnesium Transporter 1 protein (MAGT1).6 CAEBV, however, typically develops in immunocompetent patients, following primary EBV infection. It most often occurs in children and adolescents without a history of immunodeficiency or autoimmunity. The pathophysiological mechanisms of this mysterious disease remain unclear. A decrease in the number of EBV-specific cytotoxic T cells or their impaired function has been reported, which could explain the sustained EBV infection of T or NK cells in CAEBV.7 Constitutive activation of STAT3 (signal transducer and activator of transcription 3) and NF-kB (nuclear factor-kappa B) is detected in the infected cells,8, 9 which could contribute to the development of both inflammatory and neoplastic aspects of CAEBV. Recently, genomic analysis revealed high frequencies of somatic mutations, including DDX3X and KMT2D genes, which are assumed to be driver mutations.10 Intragenic deletions within the EBV genome, in the BamHI-A rightward transcripts region and in lytic genes, are found to be associated with CAEBV.10
CAEBV is a progressive disease associated with high mortality and morbidity. Cardiovascular complications may occur in CAEBV patients during the disease course. According to a nationwide survey in Japan, coronary artery aneurysms develop in 9% and myocarditis in 6% of CAEBV patients.11 Ishihara et al. reported that 17.9% of pediatric patients with CAEBV developed complications involving the circulatory system, and most of the cases consisted of coronary artery aneurysms.12 No previous reports in the existing literature have highlighted cases of CAEBV associated with multi-systemic aneurysms involving coronary arteries, abdominal arteries, cerebral arteries and vertebral arteries. High EBV load, fever, and cytopenias may be risk factors for the development of cardiovascular complications. Two pathological mechanisms of cardiovascular lesions have been considered: one is high levels of inflammatory cytokines, and the other is EBV-infected lymphocytes infiltration in the vessel walls. Kikuta et al. suggested that EBV-infected cells may play a direct pathogenic role in the development of cardiovascular complications in CAEBV, by detecting the EBV genome in cardiac and aortic tissues in three patients with CAEBV-associated coronary artery aneurysms.13 Muneuchi et al. suggested that the EBV-infected CD8+ T cells may be the major infiltrating cells in the cardiovascular lesions associated with CAEBV, and that these cells contribute to the tissue necrosis and vascular damage by the enhanced release of granzyme B and perforin.14 Moreover, EBV specific genes, such as LMP1, up-regulate pro-inflammatory and Th-1-type cytokines, leading to the secondary activation of macrophages. These cytokines would also activate endothelial cells to promote cell adhesion. Recent findings on CAEBV indicate that the upregulated expression of vascular cell adhesion molecule-1 (VCAM-1) on cytokine-stimulated endothelial cells would promote the adhesion of EBV-positive NK cells, and might initiate the vascular lesions.15
CNS vasculitis is exceptional in CAEBV patients. Kobayashi et al. reported an autopsy case of CAEBV with CNS lesions showing perivascular CD3+ and EBER-1 lymphocyte infiltration.16 Cerebral aneurysms can remain asymptomatic for a long period of time and suddenly become complicated by a fatal hemorrhagic rupture, as what happened to our patient. Early diagnosis and close monitoring is therefore essential in all CAEBV patients.
Visceral arteries aneurysms have never been reported in CAEBV patients until now. They are probably underdiagnosed because of their insidious nature. Abdominal CT Scan or angiography should be performed in all CAEBV patients.
We believe that the “multi-systemic aneurysmal disease” observed in our patient could be explained by high EBV load and cytokine storm associated with HLH. Immunophenotyping analysis of peripheral blood lymphocytes revealed the presence of an increased number of double negative T cells. In healthy individuals, these cells represent a small subpopulation of approximately 1–3% of all CD3+ T cells in the peripheral blood. Previous studies described their regulatory role in reducing immune activation during inflammation and autoimmunity.17 However, the majority of cells within the double negative T cells compartment display the phenotype of terminally differentiated effector cells that are capable of pro-inflammatory cytokine production.18 Furthermore, activated double negative T cells produce perforin which is typically expressed in cytotoxic lymphocytes.19 Recent studies reported the role of double negative T cells in response to viral infections, especially in HIV infection. However, report on their association with EBV infection is rare. We believe that double negative T cells found in the peripheral blood of our patient are involved in systemic inflammation and tissue damage. Thus, additional studies are needed to investigate the causal relationship between CAEBV and this rare and heterogeneous T lymphocyte subset.
On the other hand, EBV is reportedly linked with various forms of vasculitis, including leucocytoclastic vasculitis, granulomatous vasculitis, Kawasaki disease, and polyarteritis nodosa. Some cases of ANCA-associated vasculitis (AAV) that presented with IM were reported.20 Acute EBV infection can also trigger the relapse of disease for patients who have developed AAV.21 Since EBV infection can induce an expansion of lymphocytes, such a lymphoproliferative response could also include an expansion of the ANCA-specific lymphocyte clone.22 Further studies are therefore needed in order to prove this hypothesis.
The treatment of systemic vasculitis associated with CAEBV is challenging because of the lack of evidence about this rare disease. Many therapies, including antiviral agents, corticosteroids, intravenous immunoglobulins and chemotherapeutic drugs have been tried without obvious effect on morbidity and mortality. It was reported that bortezomib suppressed proliferation and induced apoptosis of EBV-infected T and NK cells, probably by inhibiting the NF-kB pathway.23 Currently, the only effective curative treatment strategy is allogeneic HSCT.24 Some authors reported that small coronary aneurysms regressed to normal size after successful allogeneic HSCT.14 Unfortunately, our patient died before benefiting from this therapy.
Conclusion
This case highlights an exceptional fatal complication of a rare, under-diagnosed, and little studied condition. CAEBV should be considered within the differential diagnoses while managing a patient with systemic vasculitis. Surveillance with periodic vascular imaging may be necessary in CAEBV, and future research or registry studies are needed to understand the appropriate evaluation strategy for this rarely reported disease. It is likely that the direct invasion of EBV-infected T/NK cells and the associated systemic inflammation contribute to the development of cardiovasculopathy during the progressive disease course. Further studies are needed to understand the mechanisms of organ and tissue tropism of EBV-infected cells. Early HSCT should be performed in CAEBV patients, at least before the development of life threatening complications.
Footnotes
Oumama Jamal, MD, (above), Nawal Sahel, MD, Mohammed El Qatni, PhD, Meryem Zaizaa, MD, Ilyas El Kassimi, MD, Adil Rkiouak, PhD, Salaheddine Hammi, PhD, and Youssef Sekkach, PhD, are in the Department of Internal Medicine A, Mohammed V Military Instruction Hospital, Faculty of Medicine and Pharmacy of Rabat, Mohammed V University, Rabat, Morocco. Rachinda Saouab, PhD, is in the Department of Radiology, Mohammed V Military Instruction Hospital, Faculty of Medicine and Pharmacy of Rabat, Mohammed V University Rabat, Morocco.
Disclosure
None reported.
References
- 1.Arai A. Chronic active Epstein-Barr virus infection: a bi-faceted disease with inflammatory and neoplastic elements. Immunol Med. 2018 Dec;41(4):162–169. doi: 10.1080/25785826.2018.1556030. [DOI] [PubMed] [Google Scholar]
- 2.Isaacs R. Chronic infectious mononucleosis. Blood. 1948 Aug;3(8):858–61. [PubMed] [Google Scholar]
- 3.Straus SE. The chronic mononucleosis syndrome. J Infect Dis. 1988 Mar;157(3):405–12. doi: 10.1093/infdis/157.3.405. [DOI] [PubMed] [Google Scholar]
- 4.Okano M, Kawa K, Kimura H, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005 Sep;80(1):64–9. doi: 10.1002/ajh.20398. [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 May 19;127(20):2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen JI. Primary Immunodeficiencies Associated with EBV Disease. Current topics in microbiology and immunology. 2015;390(Pt 1):241–65. doi: 10.1007/978-3-319-22822-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibayama H, Imadome KI, Onozawa E, et al. [Virus-specific cytotoxic T cells in chronic active Epstein-Barr virus infection]. Rinsho Ketsueki. 2017;58(6):583–588. doi: 10.11406/rinketsu.58.583. [DOI] [PubMed] [Google Scholar]
- 8.Cahir-McFarland ED, Carter K, Rosenwald A, et al. Role of NFkappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J Virol. 2004 Apr;78(8):4108–19. doi: 10.1128/jvi.78.8.4108-4119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kung CP, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor through effects on Bcl-3 and STAT3. J Virol. 2008 Jun;82(11):5486–93. doi: 10.1128/jvi.00125-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murata T, Okuno Y, Sato Y, Watanabe T, Kimura H. Oncogenesis of CAEBV revealed: Intragenic deletions in the viral genome and leaky expression of lytic genes. Rev Med Virol. 2020 Mar;30(2):e2095. doi: 10.1002/rmv.2095. [DOI] [PubMed] [Google Scholar]
- 11.Kimura H, Morishima T, Kanegane H, et al. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis. 2003 Feb 15;187(4):527–33. doi: 10.1086/367988. [DOI] [PubMed] [Google Scholar]
- 12.Ishihara S, Okada S, Wakiguchi H, Kurashige T, Morishima T, Kawa-Ha K. Chronic active Epstein-Barr virus infection in children in Japan. Acta Paediatr. 1995 Nov;84(11):1271–5. doi: 10.1111/j.1651-2227.1995.tb13547.x. [DOI] [PubMed] [Google Scholar]
- 13.Kikuta H, Sakiyama Y, Matsumoto S, et al. Detection of Epstein-Barr virus DNA in cardiac and aortic tissues from chronic, active Epstein-Barr virus infection associated with Kawasaki disease-like coronary artery aneurysms. J Pediatr. 1993 Jul;123(1):90–2. doi: 10.1016/s0022-3476(05)81546-x. [DOI] [PubMed] [Google Scholar]
- 14.Muneuchi J, Ohga S, Ishimura M, et al. Cardiovascular complications associated with chronic active Epstein-Barr virus infection. Pediatr Cardiol. 2009 Apr;30(3):274–81. doi: 10.1007/s00246-008-9343-8. [DOI] [PubMed] [Google Scholar]
- 15.Kanno H, Watabe D, Shimizu N, Sawai T. Adhesion of Epstein-Barr virus-positive natural killer cell lines to cultured endothelial cells stimulated with inflammatory cytokines. Clin Exp Immunol. 2008 Mar;151(3):519–27. doi: 10.1111/j.1365-2249.2007.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi Z, Tsuchiya K, Takahashi M, et al. An autopsy case of chronic active Epstein-Barr virus infection (CAEBV): distribution of central nervous system (CNS) lesions. J Neurol Sci. 2008 Dec 15;275(1–2):170–7. doi: 10.1016/j.jns.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Brandt D, Hedrich CM. TCR αβ (+)CD3(+)CD4(−)CD8(−) (double negative) T cells in autoimmunity. Autoimmun Rev. 2018 Apr;17(4):422–430. doi: 10.1016/j.autrev.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-Rodríguez N, Apostolidis SA, Fitzgerald L, et al. Proinflammatory self-reactive T cells are found within murine TCR-αβ(+) CD4(−) CD8(−) PD-1(+) cells. Eur J Immunol. 2016 Jun;46(6):1383–91. doi: 10.1002/eji.201546056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer K, Voelkl S, Heymann J, et al. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(−)CD8− doublenegative regulatory T cells. Blood. 2005 Apr 1;105(7):2828–35. doi: 10.1182/blood-2004-07-2583. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi M, Yoshioka T, Yamakawa T, et al. Anti-neutrophil cytoplasmic antibody-associated vasculitis associated with infectious mononucleosis due to primary Epstein-Barr virus infection: report of three cases. Clinical kidney journal. 2014 Feb;7(1):45–8. doi: 10.1093/ckj/sft140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schned AR, Ornvold K, Tsongalis GJ, Chobanian MC. Fatal relapse of ANCA-associated glomerulonephritis triggered by successive Epstein-Barr and varicella zoster virus infections. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006 May;47(5):915–22. doi: 10.1053/j.ajkd.2006.02.183. [DOI] [PubMed] [Google Scholar]
- 22.Xu P, Lin S, Wei L, Shang W. Antineutrophil cytoplasmic antibody-associated vasculitis associated with Epstein-Barr virus infection: a case report and review of the literature. Infection. 2014 Jun;42(3):591–4. doi: 10.1007/s15010-014-0606-4. [DOI] [PubMed] [Google Scholar]
- 23.Iwata S, Yano S, Ito Y, et al. Bortezomib induces apoptosis in T lymphoma cells and natural killer lymphoma cells independent of Epstein-Barr virus infection. Int J Cancer. 2011 Nov 1;129(9):2263–73. doi: 10.1002/ijc.25873. [DOI] [PubMed] [Google Scholar]
- 24.Sawada A, Inoue M, Kawa K. How we treat chronic active Epstein-Barr virus infection. Int J Hematol. 2017 Apr;105(4):406–418. doi: 10.1007/s12185-017-2192-6. [DOI] [PubMed] [Google Scholar]