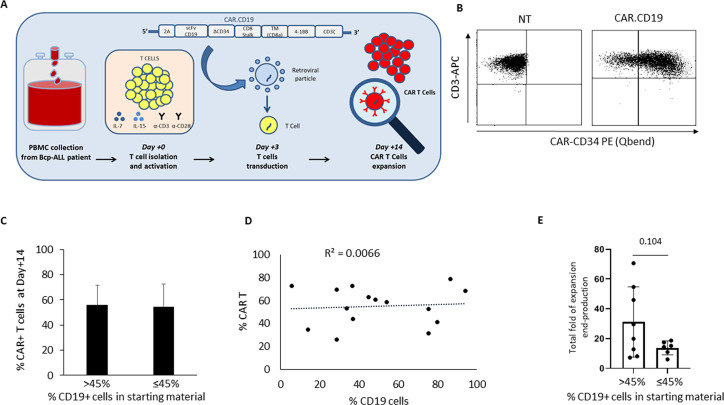
Figure 1.
CAR.CD19 T-cells generated from Bcp-ALL patients’ derived PBMCs collected at diagnosis. (A) The scFv of α-CD19 is cloned in frame with ΔCD34 trackable marker and both 4.1BB and the CD3ζ signaling endodomains. PB mononuclear cells of Bcp-ALL patients at diagnosis are activated with α-hCD3/α-hCD28 mAbs and rh-IL7/rh-IL15 and then transduced with the CAR.CD19 γ-retroviral supernatant. (B) Flow cytometry analysis of a representative donor showing CAR expression by ΔCD34 detection in untransduced (NT) T-cells (negative control; left panel) and CAR.CD19 genetically modified T-cells (right panel). (C) Percentage of CAR+ T-cells at end-production (day+14) in DPs with more than 45% (n=8) or equal to/less than 45% (n=7) of CD19+ leukemia cells in the starting raw material used for CAR T-cell manufacturing. The median value of 45% was used as cut-off. (D) Correlation matrix between percentage of cCAR+ T-cells in the DPs at the end of production and the percentage of CD19+ leukemic cells in the starting raw materials from patients. (E) Histograms representing the total fold expansion from day+3 to the end of production of CAR T-cells in the two subgroups of patients with <45% or >45% of CD19+ B-cells in the SM. Bcp-ALL, B-cell precursor acute lymphoblastic leukemia; CAR T, chimeric antigen receptor T-cells’ DPs, drug products; mAbs, monoclonal antibodies; SM, starting material; NT, non-transduced.