Abstract
Background
Oropharyngeal cancer is an important public health problem. The aim of our study was to correlatep16 immunohistochemistry in oropharynx squamous cell carcinomas(OPSCC) with clinical and epidemiological features.
Material and methods
We conducted across-sectional study on patients with OPSCC treated at a single institution from 2014 to 2019. Epidemiological and clinical-pathological data were collected from medical records and a questionnaire was applied to determine alcohol consumption, smoking, and sexual behavior. The HPV status was determined by p16 immunohistochemistry.
Results
A total of 252 patients participated in the study, of these 221 (87.7%) were male. There were 81 (32.14%) p16 positive cases and 171 (67.85%) p16 negative cases. The p16positive group was significantly associated with younger patients (50–59 years), higher education level, lower clinical stage and patients who never drank or smoked. Through univariate logistic regression, we observed that female sex (OR, 3.47; 95% CI, 1.60–7.51) and higher education level (OR, 9.39; 95% CI, 2, 81–31,38) were significantly more likely to be p16 positive. Early clinical stage (AJCC8ed) was more associated with p16 positivity both in univariate (OR, 0.14; 95% CI, 0.07–0.26, p<0.001) and multivariate analysis (OR, 0.18; 95% CI, 0.06–0.49, p = 0.001).
Conclusion
This study showed that drinkers and current smokers were less likely to be p16+. Female sex, higher education level and younger age at diagnosis were associated with a higher probability of being p16+. Additionally, there was a higher proportion of patients with early clinical stage (I or II) in the p16 positive group when compared to the p16 negative group.
Introduction
Oropharyngeal cancer is an important public health problem. According to GLOBOCAN 2018 [1], it represents 0.5% (92,887) of the total number of new cancer cases and 0.5% (51,005) of the total number of cancer deaths.
Drinking and smoking are considered serious risk factors in oropharyngeal carcinogenesis [2–4]. However, in recent decades, another important etiological factor for oropharynx squamous cell carcinomas (OPSCC) has been discovered: human papillomavirus (HPV). The prevalence of HPV in oropharyngeal tumours varies according to the year of study, population studied and method of analysis. In recent decades, HPV prevalence in these tumours has been increasing globally and are found to be more prevalent in developed countries than in developing ones [5, 6].
HPV status can be determined by several methods but, clinically, immunohistochemistry for the p16 protein has shown several benefits as it is practical, simple and inexpensive [7, 8]. Several important international guidelines, such as the National Comprehensive Cancer Network [9] and the guidelines of the American College of Pathologists [10], recommend the clinical use of p16 by immunohistochemistry to characterize cases such as HPV+ or HPV−. Additionally, the staging of oropharyngeal tumours changed in the 8th edition of the AJCC [11, 12].
The aim of our study was to correlate the p16 immunohistochemistry in OPSCC with clinical and epidemiological features.
Material and methods
We conducted a cross-sectional study of 252 patients diagnosed with OPSCC, treated at a single tertiary referral institution for cancer treatment in Brazil from 2014 to 2019. Demographic data (sex, age, marital status) and clinical-pathological data (TNM clinical stage according to the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system) were obtained from medical records. Of the 252 patients in the study, 125 answered a questionnaire on sexual behavior, smoking and alcohol consumption habits.
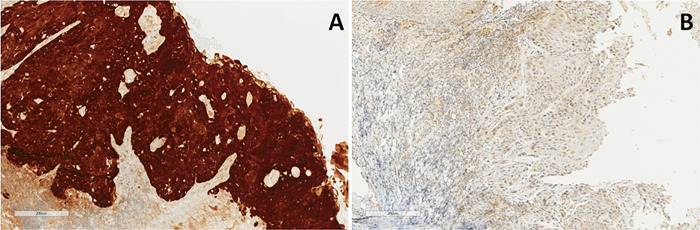
HPV status was determined in all patients by p16 immunohistochemistry, which is a well-established surrogate marker to characterize HPV+ oropharyngeal tumours [10]. Briefly, the paraffin blocks from the biopsy or surgery of the patients were separated, and the most representative areas of these blocks containing the tumours were selected and sliced into 4-μm sections. Immunohistochemistry was performed using the CINtec® p16 Histology kit (Roche MTM Laboratories, Heidelberg, Germany), according to the manufacturer’s instructions. The expression of p16 was classified as positive in the presence of strong and diffuse staining in more than 75% of both nuclei and cytoplasms. Any other colour pattern was classified as negative (Fig 1) [13–15]. Thus, patients with p16 positivity by immunohistochemistry were considered to be HPV+ [16, 17].
Fig 1. Representative photomicrograph of p16 expression (immunohistochemistry).
10x magnification. A: Oropharynx squamous cell carcinomawith p16-positive immunohistochemistry. B: Oropharynx squamous cell carcinomawith p16-negative immunohistochemistry.
Ethical approval
The study was approved by the Medical Ethics Committee of the Barretos Cancer Hospital under number 1,943,689. Written informed consent was obtained from all patients who were enrolled in this study.
Statistical analysis
The analyses were performed using the software SPSS version 21 (SPSS, Inc., Chicago, Illinois). The sociodemographic and clinical characteristics based on HPV status were analysed using the chi-squared test (or Fisher’s exact test) when the variables were qualitative and the Mann-Whitney test for quantitative variables. A p-value < 0.05 was considered statistically significant. To verify the relationships between the studied variables and HPV positivity, multivariate logistic regression was used to estimate the odds ratio (OR) and its respective 95%.
Results
In the period from 2014 to 2019, we included a total of 252 patients diagnosed with OPSCCtreated at our institution. The sociodemographic and clinical pathological characteristics are summarised in Table 1. The majority were male (221 = 87.69%), and the most frequent age group was 50–59 years, with 85 (33.7%) cases.
Table 1. Associations of p16 expression with the epidemiological characteristics of the patients.
| Variables | P16 | P-value | |||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| n | (%) | n | (%) | ||
| Sexa | |||||
| Male | 158 | 92.4% | 63 | 77.8% | 0.01* |
| Female | 13 | 7.6% | 18 | 22.2% | |
| Age b | |||||
| 20–29 | 1 | 6.1% | 0 | 0.0% | 0.005* |
| 30–39 | 1 | 6.1% | 3 | 3.7% | |
| 40–49 | 27 | 15.8% | 25 | 30.9% | |
| 50–59 | 54 | 31.6% | 31 | 38.3% | |
| 60–69 | 57 | 33.3% | 15 | 18.5% | |
| 70–79 | 22 | 12.9% | 6 | 7.4% | |
| 80–89 | 8 | 4.7% | 1 | 1.2% | |
| 90–99 | 1 | 0.6% | 0 | 0.0% | |
| Skin coloura | |||||
| White | 92 | 55.4 | 50 | 64.9 | 0.16 |
| Non-white | 74 | 44.6 | 27 | 35.1 | |
| Schoolinga | |||||
| Illiterate | 23 | 15.2% | 7 | 8.6% | 0.001* |
| Elementary school | 99 | 65.6% | 35 | 43.2% | |
| High school | 22 | 14.6% | 19 | 23.5% | |
| Higher | 7 | 4.6% | 20 | 24.7% | |
| Ta | |||||
| T1/T2 | 53 | 31.5% | 33 | 42.3% | 0.100 |
| T3/T4 | 115 | 68.5% | 45 | 57.7% | |
| Na | |||||
| N0 | 41 | 24.4% | 17 | 21.8% | 0.654 |
| N positive | 127 | 75.6% | 61 | 78.2% | |
| Mb | |||||
| M0 | 161 | 95.8% | 72 | 92.3% | 0.357 |
| M1 | 7 | 4.2% | 6 | 7.7% | |
| TNM staging a | |||||
| Early-stage I and II | 22 | 13.1 | 41 | 52.6% | 0.001* |
| Advanced III and IV | 146 | 86.9% | 37 | 47.4% | |
ª Analysis by the chi-squared test expressed as absolute (n) and relative (%) frequency
b Analysis by Fisher’s exact test expressed as absolute (n) and relative (%) frequency
* Statistically significant difference P ≤ 0.05
Data analysis showed that 30 (12.93%) patients were illiterate, 134 (57.75%) completed elementary school, 41 (17.67%) completed high school, and 27 (11, 63%) completed higher education. The results for skin colour showed that 142 (58.43%) of the patients self-reported as white while 101 (41.56%) patients self-reported as non-white (brown, black, Asian, indigenous). The findings on marital status showed that 18.79% (133/233) of the patients had a fixed partner (married or in a stable union), 21.46% (50/233) had no fixed partner (widowed or separated), and 21.46% (50/233) were single.
A total of 81 (32.1%) patients tested positive for p16 staining while 171 (67.85%) tested negative.
Although a lower number of women participated in the study, it should be highlighted that the probability of oropharyngeal cancer in women being HPV induced based on the p16 status is higher than when a man is diagnosed with oropharyngeal cancer.
In the p16+group, the patients were younger, with a predominant age range of 50–59 years compared to a predominance of 60–69 years in the other group (P = 0.005). No significant differences regarding ethnicity were observed. The p16 positive patients showed significant higher education level (24.7% vs. 4.6%, P < 0.001), and a higher proportion of patients who finished high school (23.5% vs. 14.6%, P < 0.001). In the p16 negative group, a greater proportion of individuals were illiterate (15.2% vs. 8.6%, P < 0.001).
No significant differences in relation to T, N, or M stage were identified. However, in the p16negativegroup, there was a higher number of patients with advanced clinical stage (III or IV) (86.9% vs. 47.4%) and a greater proportion of patients in the p16 positive group with early clinical stage (I or II) (13.1 vs. 52.6, P <0.001) (Table 1).
Analysis of the questionnaires on patient habits showed a much higher prevalence of patients who never drank in the p16 positive group than in the p16 negative group (16% vs. 2.7%, P = 0.015). Similarly, the number of patients who never smoked in the p16 positive group was more predominant compared to the p16 negative group (32% vs. 6.7%, P < 0.001) (Table 2).
Table 2. Data from the questionnaire related to smoking, alcohol consumption and sexual behavior.
| Variables | p16 | P-value | |||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| n | (%) | n | (%) | ||
| Alcoholb | |||||
| Never drank | 2 | 2.7% | 8 | 16.0% | 0.015* |
| Ex-drinker | 46 | 61.3% | 22 | 44.0% | |
| Current drinker | 27 | 36.0% | 20 | 40.0%% | |
| Smoking a | |||||
| Never smoked | 5 | 6.7% | 16 | 32.0% | 0.001* |
| Ex-smoker | 34 | 45.3% | 18 | 36.0% | |
| Current smoker | 36 | 48.0% | 16 | 32.0% | |
| Number of sexual partners throughout lifea | |||||
| None | 0 | 0.0% | 0 | 0.00% | 0.362 |
| 1 to 3 | 12 | 16.0% | 10 | 20.0% | |
| 4 to 10 | 27 | 36.0% | 12 | 24.0% | |
| >10 | 36 | 48.0% | 28 | 56.0% | |
| Number of partners with whom the patient practised active oral sex throughout lifeb | |||||
| None | 37 | 50.7% | 15 | 30.0% | 0.126 |
| 1 to 3 | 27 | 37.0% | 24 | 48.0% | |
| 4 to 10 | 4 | 5.5% | 5 | 10.0% | |
| >10 | 5 | 6.8 | 6 | 12.0% | |
| Number of partners with whom the patient practised passive oral sex throughout life | |||||
| None | 29 | 39.2% | 9 | 18.0% | 0.55 |
| 1 to 3 | 29 | 39.2% | 22 | 44.0% | |
| 4 to 10 | 8 | 10.8 | 11 | 22.0% | |
| >10 | 8 | 10.8 | 8 | 16.0% | |
ª Analysis by the chi-squared test expressed as absolute (n) and relative (%) frequency
b Analysis by Fisher’s exact test expressed as absolute (n) and relative (%) frequency
* Statistically significant difference
From the data, no significant differences were observed in the number of sexual partners throughout life, nor in the number of partners with whom the patients practised either passive or active oral sex (Table 2).
Through univariate logistic regression, we determined that female sex (OR, 3.47; 95% CI, 1.60–7.51) and higher education level (OR, 9.39; 95% CI, 2, 81–31,38) were significantly related to a higher probability of being p16 positive. Current alcohol drinking (OR, 0.14; 95% CI, 0.04–0.44) and current smoking (OR, 0.14; 95% CI, 0.04–0.44) were less associated with p16 positivity in univariate analysis. (Table 3). Early clinical stage was more associated with p16 positivity both in univariate (OR, 0.14; 95% CI, 0.07–0.26, p<0.001) and in multivariate analysis (OR, 0.18; 95% CI, 0.06–0.49, p = 0.001).
Table 3. Model of the univariate and multivariate analysis of sociodemographic data based on p16 status.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Odds ratio | Confidence Interval for Odds Ratio | p-value | Odds ratio | Confidence Interval for Odds Ratio | p-value | ||
| Lower | Higher | Lower | Higher | |||||
| Sex | ||||||||
| Male | 1 | - | - | 1 | - | - | ||
| Female | 3.473 | 1.606 | 7.506 | 0.002 | 3.872 | 0.808 | 18.558 | 0.09 |
| Alcohol | ||||||||
| Never drank | 1 | - | - | 0.032 | 1 | - | - | 0.227 |
| Ex-drinker | 0.120 | 0.023 | 0.611 | 0.011 | 0.297 | 0.037 | 2.356 | 0.25 |
| Current drinker | 0.186 | 0.186 | 0.035 | 0.046 | 0.593 | 0.065 | 5.372 | 0.642 |
| Smoking | ||||||||
| Never smoked | 1 | - | - | 0.003 | 1 | - | - | 0.246 |
| Ex-smoker | 0.165 | 0.052 | 0.525 | 0.002 | 0.283 | 0.064 | 1.242 | 0.094 |
| Current smoker | 0.139 | 0.043 | 0.445 | 0.001 | 0.354 | 0.077 | 1.62 | 0.181 |
| Education | ||||||||
| Illiterate | 1 | - | - | < 0,001 | 1 | - | - | 0.295 |
| elementary school | 1.182 | 0.458 | 2.943 | 0.752 | 1.537 | 0.451 | 5.234 | 0.492 |
| high school | 2.838 | 0.998 | 8.071 | 0.051 | 2.984 | 0.652 | 13.665 | 0.159 |
| higher | 9.388 | 2.808 | 31.385 | < 0,001 | 4.3 | 0.716 | 25.837 | 0.111 |
| CS | ||||||||
| Early-stage | 1 | - | - | - | 1 | - | - | |
| Advanced | 0.136 | 0.072 | 0.256 | < 0,001 | 0.182 | 0.067 | 0.492 | 0.001 |
Reference: p16+
Discussion
In the present study, we found that p16 positive oropharyngeal squamous-cell carcinoma was more common in patients who never drank, never smoked, in those with a higher educational level and was associated with tumours presenting in early clinical stage (I and II).
The majority of studies relating HPV to OPSCC do not follow standardised methods and, consequently, several authors use different diagnostic methods for HPV (p16, PCR), resulting in varied prevalence rates. In addition, the prevalence depends on the population studied and the year of the study. In this research, we considered p16 immunohistochemistry as a marker for HPV status in all patients.
One of the differentials of our study is the application of a questionnaire on sexual behavior and consumption of alcohol and tobacco. Although a difference between p16 positive and p16 negative patients regarding sexual behavior was not detected, some studies demonstrate the opposite.D’Souza et al. [18] determined that a history of 6 or more oral sex partners was associated with a greater risk of OPSCC, D’Souza et al. found that a number of vaginal sex partners (≥26 compared to 0–5) was associated with a greater risk of OPSCC among men and the studies of Baumeister et al. [19] and Dahlstrom et al. [20] found the same result. Our findings are corroborated by other authors: Talamini et al. [21], Garrote et al. [22] and Smith et al. [23] who did not find a significant association between lifetime number of sexual partners and the risk of oral cancer.
Our study showed a 32.14% prevalence of p16 positivity in oropharyngeal carcinomas. Most studies involving populations from developing countries identified a low incidence of HPV in oropharyngeal tumours [24, 25]. López et al. [26] reported an incidence of 6.6% in Brazil and Anantharaman et al. [6] compared cases from the USA, Europe, and Brazil and found an incidence of only 4.1% of positivity in the population studied in Brazil. Petito et al. [16] studied 82 cases in Brazil and found an incidence of 25.6%, Betiol et al. [27] found 17.7% and de Cicco et al. [28] reported 59.1%. A more recent study in a developing country was that of Bahl et al. [29] who found a 22.8% incidence of HPV in oropharyngeal tumours in India. In developed countries, Nasman et al. [30] studied 98 patients with oropharyngeal cancer in Sweden and found an HPV incidence of 79%.
Most of the patients in our study, in both p16+ and p16− groups, had advanced clinical stage (III or IV). However, specifically in the p16+ group, there was a proportionally larger number of patients with early clinical stages (I and II) compared to the p16− group (52.6% vs. 13.1%, P < 0.001). De Cicco et al. [28] and Du et al. [31] reported similar results.
Based on the biological behavior of induced HPV oropharyngeal cancer, there was a change in staging in the eighth edition, generating its own staging for the positive p16. This made it possible to perform a downstage of this population in comparison with the negative p16. Therefore, what is seen in the results are tumours that are now considered to be initial due to the new staging, which is inherent in the staging, which better reflects the positive p16 neoplasia and its consequent better prognosis.
According to Vokes et al. [32] this difference in prognosis by p16 immunohistochemical status was considered in the change in staging that occurred between the 7th and 8th edition of the TNM. In the seventh, only T1 or T2 N0 and M0 were considered early-stagetumours, regardless of their HPV status. In the eighth edition, the HPV-negative tumours continued to be considered early stage T1 or T2 N0 and M0, but among the HPV+ tumors, T3 and N2 were also considered early stage. Although the 8th edition of the TNM staging manual separates the staging of oropharyngeal squamous-cell carcinomas according to HPV positivity, the treatment for HPV-associated oropharyngeal carcinomas remains the same for HPV−tumours, except in clinical trials [32].
In Table 4, we observe the differences between the 7th and 8th edition of the TNM (Table 4) and in particular that in the positive p16 all T3 / N2 are stage II, all T4 / N3 are stage III and stage IV only for M1.
Table 4. Major changes from the seventh to the eighth editions of TNM [12].
| Edition of TNM | Main characteristics |
|---|---|
| 7Th edition | 1-presence of T0 and Tis |
| 2-no emphasis on extra-capsular extension | |
| 3-division of T4 into a and b | |
| 4-stage IV for disease T4, N2, N3 and M1 | |
| 5-number of metastatic lymph nodes differed from N2a to N2b | |
| 8Th edition | 1-absence of T0 in negative p16 and absence of Tis for positive p16 |
| 2-creation of extra-capsular extension (N3b) only for 16 negatives | |
| 3-absence of T4b for positive p16 | |
| 4-stage IV only for M1 disease in positive p16 5-without distinction of number of lymph nodes in positive p16 |
In both the univariate and multivariate analyses in our study, patients with advanced clinical stages (III and IV) were less likely to be p16+. This finding does not corroborate with that of Mehanna et al. [33]. On the other hand, no association was observed in our study between tumour size or the presence of cervical or distant metastases and positivity to p16. Our findings corroborate those found by López et al. [26]
Our findings showed a greater predominance of patients who had never smoked in the p16+ group than in the p16−group (32% vs. 6.7%, P <0.001). López et al. [26] reported a proportion of 14.3% of patients who never smoked among the HPV+ patients and 3.4% among the HPV−patients. Additionally, a higher proportion of current smokers among HPV− patients was observed(69.8% vs. 57.1%). The study by Bahl et al. [29] also identified a higher proportion of patients who had never smoked in the HPV+ group (17% vs. 11%), but this was not statistically significant (p = 0.86). Other studies have also found a difference in the prevalence of p16 immunohistochemistry positivity with smoking status [24, 28]. In clinical practice, we observed that this difference is explained by the etiology of HPV− cases, most of which are related to drinking and smoking.
Although we only analysed 125 of the 252 patients who answered the questionnaire on smoking habits, alcohol consumption, and sexual behavior, the smoking and alcohol consumption variables were statistically significant. There was a higher prevalence of patients who never drank in the p16+ group than in the p16− group (16% vs. 2.7%, P = 0.015). De Cicco et al. [28] and López et al. [26] found similar results. In comparison, Bahl et al. [29] did not find evidence of a correlation between alcohol consumption and HPV positivity (P = 0.18).
There was a greater predominance of males in the p16− group (92.4% vs. 77.8%, P = 0.01) and this finding is in agreement with those reported by López et al. [26] Petito et al. [16] the ICON-S study [34], Bahlet al. [29] and De Cicco et al. [28]. In most cases, males are more likely to have tumours of the head and neck, mainly due to their greater exposure to alcohol and smoking compared to women. The same result was found in the in the p16− group.
Although most patients were male in both the HPV+ and HPV-groups, the probability of a woman being HPV+ was greater than being HPV- negative.
The age of the patients in the study ranged from 26 to 98 years, with a mean of 60.5 years. The most frequent age group was 50–59 years, with 85 (33.73%) patients. In the p16+ group, the patients were younger, with a predominant age range of 50–59 years vs. 60–69 years in the p16−group (P = 0.005). The data of our study is in agreement with the worldwide literature which shows that HPV+ tumours affect younger patients [16, 29]. In contrast, de Cicco did not find significant differences in age between HPV+ and HPV− patients [28].
As regards education level, the group with p16+ immunohistochemical examination had more patients with higher education(24.7% vs. 4.6%, P < 0.001) and patients with a high-school education (23.5% vs. 14.6%, P < 0.001). In the p16− group, there was a greater proportion of individuals who were illiterate or who could only read and write (15.2% vs. 8.6%, P < 0.001). Our results corroborate worldwide literature which correlates HPV+ oropharyngeal tumours with patients with higher educational levels [35, 36].
The following variables were not statistically significant in our study: number of sexual partners throughout life and number of partners with whom the patients practiced either passive or active oral sex. These findings are not in accordance with the current literature, where sexual behavior is correlated with HPV positivity [10, 29, 37–41]. It is possible that our patients were more reticent about answering questions on sexual behavior due to embarrassment or shyness.
In our study we found that drinkers and current smokers had a lower chance of having a p16+ immunohistochemical test. Female sex and higher education level were associated with a higher probability of a p16+ immunohistochemical test. We did not find any correlation between the number of sexual partners throughout life or number of partners with whom the patient practiced oral sex (passive or active) throughout life and p16+ immunohistochemical test. These results are relevant because they demonstrate that p16-positive oropharyngeal tumours have a different epidemiological profile. Based on its biological behavior and clinical presentation, HPV+ oropharyngeal tumour is a pathology that requires further study and broader understanding in order to better optimize and stratify the care and treatment of patients with oropharyngeal cancer.
Supporting information
(XLSX)
Acknowledgments
We thank Barretos Cancer Hospital. We are also thankful to the Nucleus of Epidemiology and Statistics of Barretos Cancer Hospital for its support in statistical analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
There is no specific fund that financed the study.
References
- 1.Bray F., et al., Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018. 68(6): p. 394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.INCA, Estimatimativa 2020: incidência de câncer no Brasil / Jose Alencar Gomes da Silva instituto nacional do câncer. 2019, Rio de Janeiro, RJ: INCA. [Google Scholar]
- 3.Wyss A., et al., Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the international head and neck cancer epidemiology consortium. Am J Epidemiol, 2013. 178(5): p. 679–90. doi: 10.1093/aje/kwt029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorphe P., et al., Smoking and papillomavirus DNA in patients with p16-positive N3 oropharyngeal squamous cell carcinoma. Head Neck, 2019. 41(4): p. 1039–1045. doi: 10.1002/hed.25523 [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi A.K., et al., Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol, 2011. 29(32): p. 4294–301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anantharaman D., et al., Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Cancer, 2017. 140(9): p. 1968–1975. doi: 10.1002/ijc.30608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson M., et al., HPV specific testing: a requirement for oropharyngeal squamous cell carcinoma patients. Head Neck Pathol, 2012. 6 Suppl 1(Suppl 1): p. S83–S90. doi: 10.1007/s12105-012-0370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fakhry C., et al., Human papillomavirus testing in head and neck carcinomas: ASCO clinical practice guideline endorsement of the college of American pathologists guideline. J Clin Oncol, 2018. 36(31): p. 3152–3161. doi: 10.1200/JCO.18.00684 [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Head and neck cancer (version 2.2020). 2020. Agost 18, 2020]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. [DOI] [PubMed] [Google Scholar]
- 10.Lewis J.S., et al., Human papillomavirus testing in head and neck carcinomas: guideline from the college of American pathologists. Arch Pathol Lab Med, 2018. 142(5): p. 559–597. doi: 10.5858/arpa.2017-0286-CP [DOI] [PubMed] [Google Scholar]
- 11.Lydiatt W., Ridge J., and Patel S., Oropharynx (p16-) and hypopharynx, in AJCC cancer staging manual, Amin M., Editor. 2017, Springer: New York. p. 123. [Google Scholar]
- 12.O’Sullivan B., Lydiatt W., and Haughey B., HPV-mediated (p16+) oropharyngeal cancer, in AJCC cancer staging manual, Amin M., Editor. 2017, Springer: New York. p. 113. [Google Scholar]
- 13.Jordan R.C., et al., Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol, 2012. 36(7): p. 945–54. doi: 10.1097/PAS.0b013e318253a2d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen C.G., et al., Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: a systematic review. Br J Cancer, 2014. 110(6): p. 1587–94. doi: 10.1038/bjc.2014.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Possati-Resende J.C., et al., The accuracy of p16/Ki-67 and HPV test in the detection of CIN2/3 in women diagnosed with ASC-US or LSIL. PLoS One, 2015. 10(7): p. e0134445. doi: 10.1371/journal.pone.0134445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petito G., et al., Human papillomavirus in oral cavity and oropharynx carcinomas in the central region of Brazil. Braz J Otorhinolaryngol, 2017. 83(1): p. 38–44. doi: 10.1016/j.bjorl.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posner M.R., et al., Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol, 2011. 22(5): p. 1071–1077. doi: 10.1093/annonc/mdr006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM et al. Case-control study of human papillomavirus and oropharyngeal cancer. New England J Med 2007;356:1944–56. doi: 10.1056/NEJMoa065497 [DOI] [PubMed] [Google Scholar]
- 19.Baumeister P, Reiter M, Welz C, Becker S, Betz C, Harreus U. Surgically treated oropharyngeal cancer: risk factors and tumor characteristics. J Cancer Res ClinOncol2014;140:1011–9. doi: 10.1007/s00432-014-1631-5 [DOI] [PubMed] [Google Scholar]
- 20.Dahlstrom KR, Li G, Tortolero-Luna G, Wei Q, Sturgis EM. Differences in history of sexual behavior between patients with oropharyngeal squamous cell carcinoma and and patients with squamous cell carcinoma at other head and neck sites. Head Neck 2011;33:847–55. doi: 10.1002/hed.21550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talamini R, Vaccarella S, Barbone F, Tavani A, La Vecchia C, Herrero R et al. Oral hygiene, dentition, sexual habits and risk of oral cancer. Br J Cancer 2000;83:1238–42 doi: 10.1054/bjoc.2000.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrote LF, Herrero R, Reyes RM, Vaccarella S, Anta JL, Ferbeye L et al. Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer 2001;85:46–54. doi: 10.1054/bjoc.2000.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope 1998;108:1098–103 doi: 10.1097/00005537-199807000-00027 [DOI] [PubMed] [Google Scholar]
- 24.Mehanna H., et al., Geographic variation in human papillomavirus-related oropharyngeal cancer: data from 4 multinational randomized trials. Head Neck, 2016. 38 Suppl 1(Suppl 1): p. E1863–E9. doi: 10.1002/hed.24336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caley A., et al., Multicentric human papillomavirus-associated head and neck squamous cell carcinoma. Head Neck, 2015. 37(2): p. 202–8. doi: 10.1002/hed.23584 [DOI] [PubMed] [Google Scholar]
- 26.Lopez R.V., et al., Human papillomavirus (HPV) 16 and the prognosis of head and neck cancer in a geographical region with a low prevalence of HPV infection. Cancer Causes Control, 2014. 25(4): p. 461–71. doi: 10.1007/s10552-014-0348-8 [DOI] [PubMed] [Google Scholar]
- 27.Betiol J.C., et al., Prevalence of human papillomavirus types and variants and p16(INK4a) expression in head and neck squamous cells carcinomas in Sao Paulo, Brazil. Infect Agent Cancer, 2016. 11: p. 20. doi: 10.1186/s13027-016-0067-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Cicco R., et al., Impact of human papillomavirus status on survival and recurrence in a geographic region with a low prevalence of HPV-related cancer: a retrospective cohort study. Head Neck, 2020. 42(1): p. 93–102. doi: 10.1002/hed.25985 [DOI] [PubMed] [Google Scholar]
- 29.Bahl A., et al., Prevalence and trends of human papillomavirus in oropharyngeal cancer in a predominantly north Indian population. Head Neck, 2014. 36(4): p. 505–10. doi: 10.1002/hed.23317 [DOI] [PubMed] [Google Scholar]
- 30.Nasman A., et al., Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer, 2009. 125(2): p. 362–6. doi: 10.1002/ijc.24339 [DOI] [PubMed] [Google Scholar]
- 31.Du E., et al., Long-term Survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and Human Papillomavirus Status. Laryngoscope, 2019. 129(11): p. 2506–2513. doi: 10.1002/lary.27807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vokes E.E., Agrawal N., and Seiwert T.Y., HPV-associated head and neck cancer. J Natl Cancer Inst, 2015. 107(12): p. djv344. doi: 10.1093/jnci/djv344 [DOI] [PubMed] [Google Scholar]
- 33.Mehanna H., et al., Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta-analysis of trends by time and region. Head Neck, 2013. 35(5): p. 747–55. doi: 10.1002/hed.22015 [DOI] [PubMed] [Google Scholar]
- 34.O’Sullivan B., et al., Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol, 2016. 17(4): p. 440–451. doi: 10.1016/S1470-2045(15)00560-4 [DOI] [PubMed] [Google Scholar]
- 35.Gillison M.L., et al., Prevalence of oral HPV infection in the United States, 2009–2010. JAMA, 2012. 307(7): p. 693–703. doi: 10.1001/jama.2012.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahlstrom K.R., et al., Socioeconomic characteristics of patients with oropharyngeal carcinoma according to tumor HPV status, patient smoking status, and sexual behavior. Oral Oncol, 2015. 51(9): p. 832–8. doi: 10.1016/j.oraloncology.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro K.B., et al., Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. Int J Epidemiol, 2011. 40(2): p. 489–502. doi: 10.1093/ije/dyq249 [DOI] [PubMed] [Google Scholar]
- 38.Gillison M.L., et al., Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst, 2008. 100(6): p. 407–20. doi: 10.1093/jnci/djn025 [DOI] [PubMed] [Google Scholar]
- 39.D’Souza G., et al., Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med, 2007. 356(19): p. 1944–56. doi: 10.1056/NEJMoa065497 [DOI] [PubMed] [Google Scholar]
- 40.D’Souza G., et al., Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis, 2009. 199(9): p. 1263–9. doi: 10.1086/597755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Read T.R., et al., Oral human papillomavirus in men having sex with men: risk-factors and sampling. PLoS One, 2012. 7(11): p. e49324. doi: 10.1371/journal.pone.0049324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.