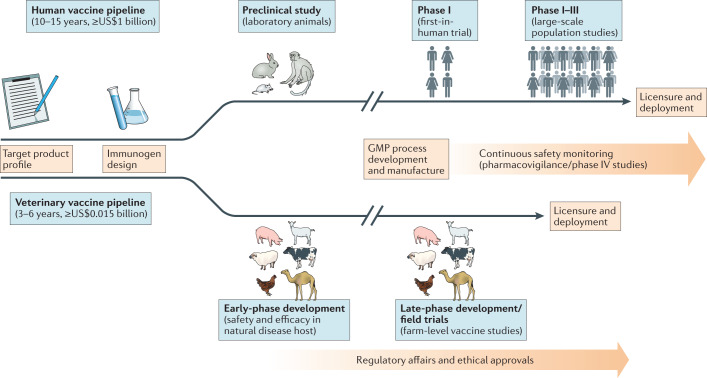
Fig. 1. Vaccine development pipeline.
The typical vaccine development pipeline is shown, starting from target product profiling to licensure and deployment. The respective stages and approximate costs for veterinary and human vaccines are shown. Although presented as a linear chronological process, some of the different stages of the pipeline for a ‘multispecies’ vaccine can occur in parallel. For instance, the candidate ChAdOx1 RVF vaccine against Rift Valley fever12 will soon undergo evaluation in human clinical trials in parallel with veterinary development, having been made with the same manufacturing starting material. GMP, good manufacturing practice.