Figure 5. Mucus structure and synthesis.
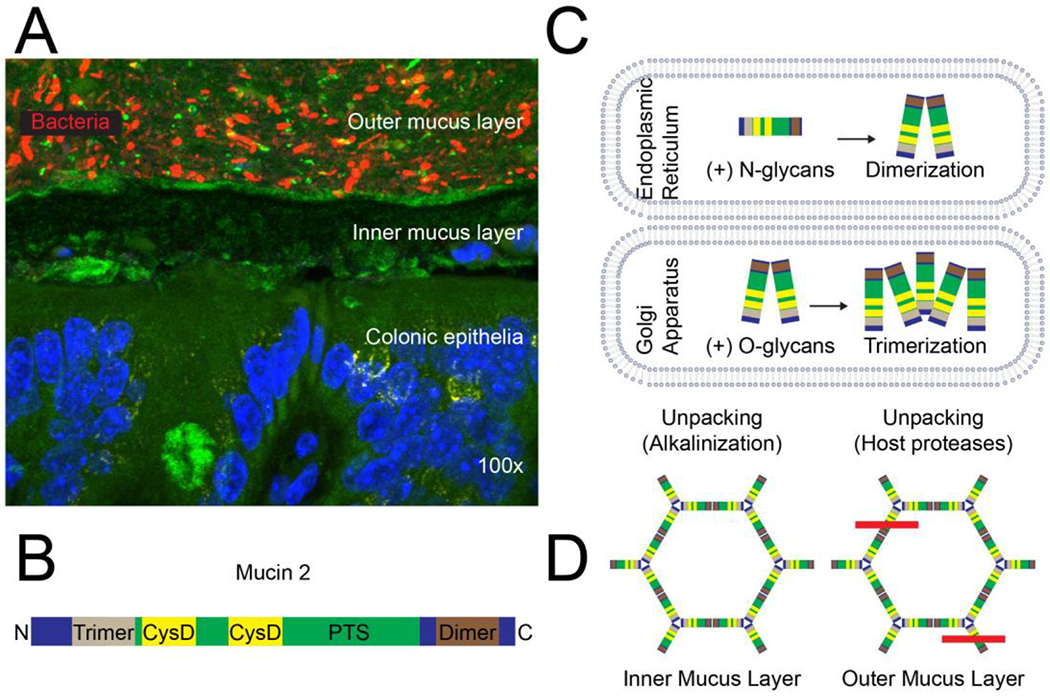
(A) Topography of the normal colonic mucus layer. Bacteria in the outer mucus layer overlay the inner mucus layer, which is normally impenetrable to bacteria; DAPI (blue), MUC2 (green; sc-15334, H-300), bacteria (16S FISH; red), magnification: 100x15. (B) The MUC2 glycoprotein has an N-terminal trimerization and a C-terminal dimerization domain with a PTS domain that is rich in proline, threonine, serine in between. The N-terminus contains three von Willebrand D domains and the C-terminus contains one (not shown). The PTS domain is highly O-glycosylated in the Golgi and interspersed by two CysD domains, contributing to intramolecular disulfide bonds and intermolecular non-covalent interactions. (C) MUC2 is synthesized in the endoplasmic reticulum, N-glycosylated and dimerized. It is then transported to the Golgi through an N-glycan-dependent mechanism, where it is O-glycosylated and forms trimers. (D) MUC2 is released and unpacked in the inner mucus layer through alkalinization and chelation of Ca2+ ions and then further unpacked in the outer mucus layer through proteolysis; the red line indicates cleavage by host proteases.
