Fig. 3.
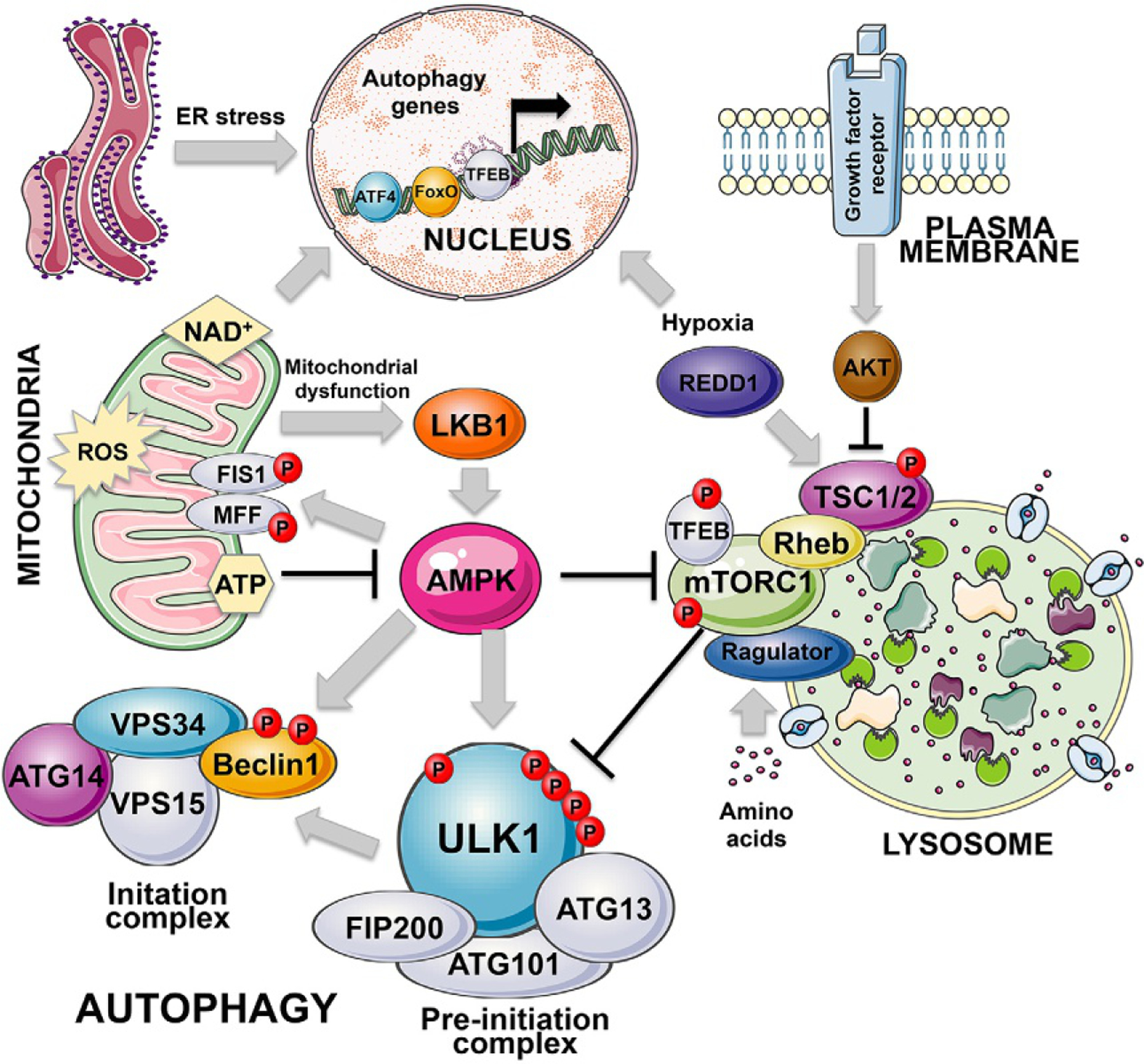
Posttranslational control of autophagy. The key posttranslational regulators of autophagy are AMPK and mTOR, kinases that sense the nutrient status of the cells to either inhibit or promote cell growth and conversely to either activate or inhibit autophagy as an adaptive response to nutrient stress. AMPK is activated by high AMP levels that arise when ATP levels are low, either due to the low energy state of the cell in general or due to mitochondrial dysfunction that can cause low ATP levels. LKB1 is a critical upstream regulator of AMPK, that is itself activated by mitochondrial dysfunction, including inhibition of the electron transport chain. AMPK in addition to promoting general autophagy by phosphorylating ULK1/2 and also Beclin1, promotes mitophagy via phosphorylation and activation of FIS1 and MFF, both receptors for DRP1 in mitochondrial fission, but emerging data suggests FIS1 plays a role in integrating mitochondrial remodeling with the autophagy machinery. mTOR by contrast with AMPK inhibits autophagy downstream of major growth stimulatory signals including growth factor receptor stimulation and AKT activation. Nutrient deprivation, including hypoxia, promotes autophagy via mTOR inhibition, mediated by specific regulators including REDD1 that is a HIF1 target. The mitochondria is a major signaling organelle that can induce mitophagy via production of reactive oxygen species (ROS), or suppress autophagy via sustained ATP production that prevents AMPK activation. Mitochondria also contribute to cellular redox balance by maintaining levels of NAD+ that keep Sirtuins in an active conformation ensuring activity of FoxOs and low PARP activity.
