Abstract
This manuscript summarizes the results of the consensus meeting composed of hematologists and cardiologists to establish recommendations for the prevention and follow-up of cardiovascular (CV) risk in patients with chronic myeloid leukemia (CML) treated with BCR-ABL tyrosine kinase inhibitors (TKIs) from the point of view of clinical practice and from the perspective of hematology consultation.
In the first medical appointment, the CV risk factors should be identified to perform the baseline risk stratification, based on the Brazilian Guideline of Dyslipidemia and Atherosclerosis Prevention Update (risk levels: very high, high, intermediate and low).
Once stratified, the treatment of the CV risk factors should be administered. If the patient presents risk factors, such as hypertension, diabetes, renal disease, smoking and hypercholesterolemia, the evaluation and initial treatment may be done by the hematologist, being an option the request for evaluation by a specialist. If the patient has a history of previous CV disease, we recommend referral to a specialist.
As the CV risk score is dynamic and the control of risk factors can reduce the patient risk, this expert consensus recommends that the re-evaluation of the CV risk after the baseline should be performed at 3 months, 6 months and 12 months. After this period, it should be done annually and, for specific patients, at the clinician’s discretion.
The evaluation of the baseline CV risk and the safe administration of a TKI allow the patient to benefit from the maximum treatment, avoiding unwanted effects.
Keywords: Cardiovascular diseases; Leukemia, myeloid; Protein kinase inhibitors; Risk factors; Risk management
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative neoplasm, characterized by the presence of the BCR-ABL1 gene fusion, accounting for 15–20% of all leukemias.1 The estimated annual incidence of CML is 1.75 cases/100,000 subjects, ranging from 0.09/100,000 in those ≤15 years old to 7.88/100,000 in the population aged ≥75 years old.2 In Brazil, about 81 thousand chemotherapy procedures for adult CML were registered in 2012, with an estimated annual prevalence of 10,125 cases.3
The management of CML has been transformed since the emergence of BCR-ABL tyrosine kinase inhibitors (TKIs), which introduced a new era in CML treatment, bringing life expectancies close to those of the general population for patients responsive to treatment.4 The first TKI released was imatinib mesylate, an inhibitor of the BCR-ABL1 and other tyrosine kinases (TKs).5, 6 Imatinib showed to be effective for CML therapy with an overall survival of 85% in an 8-year follow-up study. However, the development of resistance and intolerance represent a limitation of the imatinib treatment. Approximately 17 and 15% of patients presented primary and secondary resistance, respectively.7 The introduction of second generation TKIs (nilotinib and dasatinib) was effective in approximately 50% of those with imatinib resistance or intolerance.8, 9, 10, 11 Concerning the intolerance, in a 3-year median time, 17.5% of the patients discontinued imatinib after adverse drug reactions.12 Other drugs with evidence of efficacy are already prescribed in the international market, such as ponatinib and bosutinib.13, 14 However, this recommendation will only cover drugs licensed for marketing in Brazil according to national regulatory agencies.
The TKs are a family of enzymes that catalyze the transfer of phosphate from adenosine triphosphate to tyrosine residues specific proteins. They act in eukaryotic cellular signaling and their dysregulation has been associated with multiple types of cancer, including CML, becoming hyperactive by several means.15 The TKs also play a critical role in the cardiovascular (CV) system, including vascular, metabolic and myocardial physiology. Thus, the inhibition of certain TKs can interfere in the CV system function and cause clinical complications.16 The initial observation of CV events in patients with CML on TKIs occurred in clinical trials and in the real-world scenario, and not in experimental studies. To date, the mechanism by which cancers and the cardiovascular system share common pathways is not fully understood.16
All approved TKIs for CML treatment have activity against the BCR-ABL1. However, they present different potency and activity against the BCR-ABL1 and other kinases, including those involved in the cardiovascular system, leading to a diversity of cardiovascular toxic effects reported with different TKIs.17, 18, 19
Previous meta-analysises indicated that second generation TKIs have been associated with increased risk of vascular occlusive events, when compared to imatinib, in patients with CML.20 Similarly, in a population-based cohort study, the event rates for myocardial infarction were higher in patients treated with second-line generation TKIs (19–29 per 1000 person-years) than in those receiving first-generation TKIs (8 per 1000 person-years). Among TKI users that developed myocardial infarction, 84% had at least one major cardiac risk factor detected before the event.21
Nilotinib has been associated with vascular toxicity.18 In a retrospective multicenter analysis, 6.2% of the patients developed peripheral arterial disease.22 In a meta-analysis, peripheral arterial occlusive disease (PAOD) was reported in 1.3% of those treated with nilotinib versus 0.6% of the patients treated with no TKI and 0.2% of the patients treated with imatinib.23 In a 5-year follow-up study, CV events, namely, ischemic heart disease, ischemic cerebrovascular events and/or peripheral artery disease were reported in 7.5%, 13.4% and 2.1% of the patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily and imatinib arms, respectively.24
Dasatinib has a potential to cause pulmonary arterial hypertension (PAH).11 In a 5-year follow-up of the DASISION study, PAH was reported in 5% of the patients on dasatinib and 0.4% of those on imatinib, evidencing a continuous risk. Drug-related pleural effusion was more common with dasatinib (28%) than with imatinib (0.8%).25
Importantly, most CV events observed in patients treated with TKIs occurred in individuals with a baseline moderate-to-high CV risk profile due to a history of previous CV risk factors.26 In addition to the CV system damage caused by the TKIs, the baseline CV risk of CML patients appears to be higher than that of the general population. A real-world study evaluated the prevalence of cardiovascular disease (CVD) and CV risk factors in 1639 patients with CML in the United States. In a 5-year follow-up, the prevalence of CVD conditions was 33.0% and that of CVD risk factors was 77.7%, respectively. Compared to the general US adult population, the standardized prevalence rates at 1 year in patients with CML were significantly higher (1.3–3.5 times for CVD conditions and 20–40% significantly higher for hypertension, diabetes, and obesity; p < .001).27
In Brazil, a retrospective study evaluated the CV risk and CV events. The cohort of Brazilian patients treated with a TKI showed a 4.29% cumulative incidence of CV events in patients with CML, more frequently in those treated with second-generation TKIs. Three events occurred during dasatinib treatment, 6 during nilotinib treatment and none during imatinib treatment. Arterial occlusive events occurred in 2.6% of the patients treated with dasatinib and in 14.2% of the patients treated with nilotinib. All the events occurred in patients with high and very high cardiovascular risk.28
The increased risk of CVD observed in patients with CML eligible for TKIs treatment underscores the importance of recommendations to evaluate CV risk when selecting TKIs. This manuscript summarizes the results of the consensus meeting composed of hematologists and cardiologists who are experts in CML treatment and CV risk factors evaluation to establish recommendations for the prevention and follow-up of CV risk in patients with CML treated with TKIs from the point of view of clinical practice and from the perspective of hematological consultation.
Risk assessment and stratification
During the anamnesis, in addition to the questions usually asked about CML, it is recommended to investigate family or personal history of CVD and/or risk factors for CVD, as detailed in Table 1. Likewise, complementary exams should be requested for baseline CV risk investigation, in addition to those usually requested for the treatment of the patient with CML, and are specified in Table 1.
Table 1.
Suggested CV evaluation during the first medical appointment before initiating TKI treatment in CML patients: anamnesis, physical exam and complementary exams.
| Anamnesis | |
|---|---|
| Active CVD | Necessary |
| Previous episodes of CVD | Necessary |
| Family history of premature CVDa | Necessary |
| CVD attributable to pharmacological toxicity | Necessary |
| Drug treatment for CVD | Necessary |
| Intermittent claudication | Necessary |
| Chronic pulmonary diseases | Necessary |
| Recurrent respiratory infections | Necessary |
| Weight, height and body mass index | Necessary |
| Diagnosed diabetes mellitus | Necessary |
| History of hypertension | Necessary |
| Hypercholesterolemia | Necessary |
| CVD related symptoms | Necessary |
| Smokingb | Necessary |
| Complementary exams | |
| Blood glucose | Necessary |
| Glycated hemoglobin | Recommended |
| Lipid panel | Necessary |
| Urea and creatinine | Recommended |
| Blood pressure measurement | Necessary |
| Electrocardiogram | Necessary |
| Echocardiogram with cardiac function | Recommended |
| Brachial ankle index | Recommended |
CVD: cardiovascular disease.
Premature CVD defined as occurring in <55 years for men and <65 years for women.
At least one cigarette in the last month.
There is no specific tool designed to estimate CVD risk, particularly in the CML population. Thus, to predict CV risk in CML patients, one should use available CVD risk scores for the general population. These scores are based on the identification of CV risk factors, so that the estimated CVD risk consists of the sum and potentiation caused by synergisms among some of these factors.29
At the first medical appointment of a patient diagnosed with CML, for whom it is desirable to start a TKI treatment, the hematologist should conduct a directed anamnesis, aiming to identify CV risk factors, request complementary exams and carry out the baseline CV risk stratification. If relevant CV symptoms are detected, the patient should be referred to a cardiologist to conduct the proper investigation.
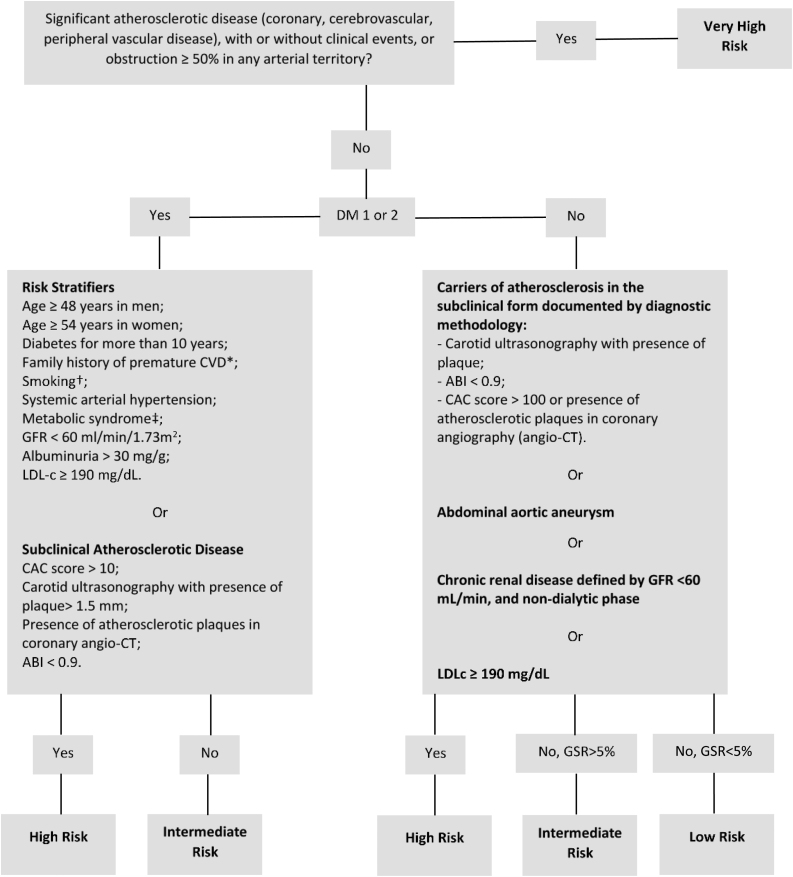
For the stratification of CV risk, the Brazilian Guideline of Dyslipidemia and Atherosclerosis Prevention Update is recommended. According to this guideline, patients can be classified into four risk levels: very high, high, intermediate and low.29
Very high risk
This is constituted by an established atherosclerotic disease (coronary, cerebrovascular, peripheral vascular disease, with or without clinical events (Grade of Recommendation: IIa; Level of Evidence: B)) or an obstruction ≥50% in any arterial territory (Grade of Recommendation: IIa; Level of Evidence: C).29 It is important to highlight that this information should be obtained by medical records, previous exams (if available) and anamnesis. In asymptomatic and stable patients, screening for atherosclerotic disease was associated with no benefits in hard outcomes and is not recommended.
High risk
Individuals considered to be at high risk are those with:
-
•
Atherosclerosis in the subclinical form documented by diagnostic methodology: carotid ultrasonography with the presence of plaque, ankle-brachial index (ABI) <0.9, coronary artery calcium score (CAC) >100 or the presence of atherosclerotic plaques in the coronary angiography (angio-CT).
-
•
Abdominal aortic aneurysm.
-
•
Chronic renal disease defined by the glomerular filtration rate (GFR) <60 mL/min. and non-dialytic phase.
-
•
Those with LDL-c concentrations ≥190 mg/dL.
-
•
Types 1 or 2 diabetes mellitus, with LDL-c between 70 and 189 mg/dL and the presence of risk stratifiers (RS) or subclinical atherosclerotic disease (SAD).
RS and SAD in diabetes are defined as:
RS: age ≥48 years in men and ≥54 years in women; time from diagnosis of diabetes >10 years; family history of a first-degree relative with premature CVD (<55 years for men and <65 years for women); smoking (at least one cigarette in the last month); hypertension; metabolic syndrome, according to the International Diabetes Federation; presence of albuminuria >30 mg/g of creatinine and/or retinopathy, and; GFR <60 mL/min.
SAD: carotid ultrasonography with the presence of plaques >1.5 mm; ABI <0.9; CAC score >10, and; the presence of atherosclerotic plaques in the coronary angio-CT.
-
•
Patients with LDL-c between 70 and 189 mg/dL, in men at risk, calculated by the Framingham-based global risk score (GRS)30 >20%, and in women >10%. (Grade of Recommendation: IIa; Level of Evidence: C).
Similar to the screening of atherosclerotic disease, the screening of SAD is not recommended for asymptomatic stable patients. Hence, the hematologist should obtain this information from medical records and clinical anamnesis. For the stratification of CV risk purposes, the lack of previous investigation of SAD should be interpreted as the absence of SAD and should not trigger complementary exams.
Intermediate risk
Males with GRS between 5% and 20% and females between 5% and 10% (Grade of Recommendation: I, Level of Evidence: A) or diabetics without the criteria of SAD or RS listed above.
Low risk
Male and female patients at risk at 10 years <5%, calculated by GRS (Grade of Recommendation: I, Level of Evidence: A).
We strongly recommend the use of the online cardiovascular risk stratification tool available for use in mobile phones and tablets with Android or IOS systems, developed by the Brazilian Society of Cardiology - Department of Atherosclerosis, Brazilian Society of Endocrinology and Metabology and the Brazilian Society of Diabetes (http://departamentos.cardiol.br/sbc-da/2015/CALCULADORAER2017/index.html). The simplified algorithm for risk stratification, adapted from the calculator for CV risk stratification is available in Figure 1.29
Figure 1.
Algorithm for cardiovascular risk stratification.
Adapted from the online cardiovascular risk stratification tool developed by the Brazilian Society of Cardiology - Department of Atherosclerosis, Brazilian Society of Endocrinology and Metabology and the Brazilian Society of Diabetes.
*Family history of a first-degree relative with premature CVD (<55 years for men and <65 years for women).
†Smoking is defined as at least one cigarette in the last month.
‡Metabolic syndrome is defined according to the International Diabetes Federation.
GSR: Global risk score, calculated depending on factors such as gender, age, systemic blood pressure, active treatment for hypertension, smoking, use of statins, total cholesterol and LDL-c.
ABI: Ankle-Brachial Index; CAC: Coronary Artery Calcium; CT: computed tomography; CVD: cardiovascular disease; DM: diabetes mellitus; GFR: Glomerular Filtration Rate.
Treatment
Once stratified, the treatment of the CV risk factors should be performed. If the patient presents risk factors, such as hypertension, diabetes, renal disease, smoking and hypercholesterolemia, the evaluation and initial treatment may be done by a hematologist, being an option the request for an evaluation by a specialist. If the patient has a history of previous CV disease, we recommend referral to a specialist. At any time, if the hematologist does not feel comfortable conducting the treatment of CV risk factors, the patient should be referred to a cardiologist or a GP with experience in CVD.
Hypertension control
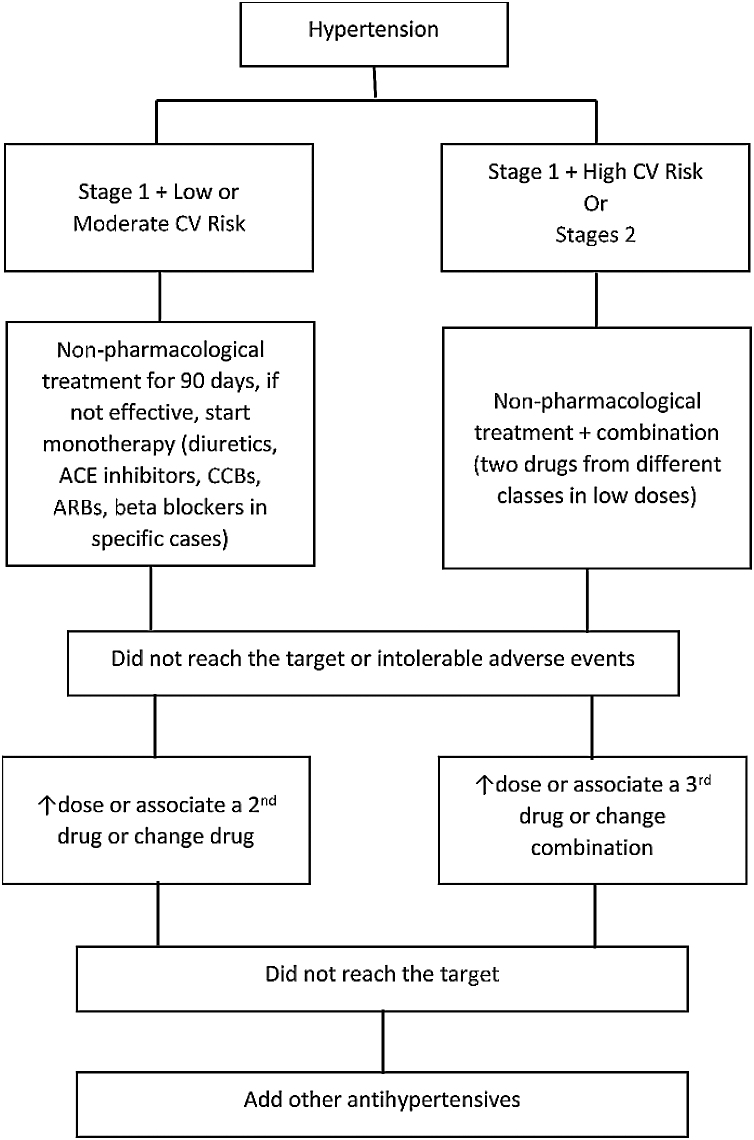
Non-pharmacological treatment of arterial hypertension involves weight control, nutritional measures, physical activity practice, smoking cessation and stress management, among others. Drug treatment is indicated for subjects with stage 1 hypertension and low or moderate CV risk, when non-pharmacological measures were not effective after an initial period of at least 90 days. For those in stage 1 and at high CV risk or with established CVD, drug use should be started immediately. Similarly, in stage 2 hypertension cases, regardless of CV risk, drug treatment should be started immediately (classification of blood pressure in adults is shown in Table 2).31 The drug treatment algorithm, adapted from the 7th Brazilian Arterial Hypertension Guideline, is presented in Figure 2.32
Table 2.
Categories of blood pressure in adults.
| Blood pressure category | Systolic blood pressure (mm Hg) | Diastolic blood pressure (mm Hg) | |
|---|---|---|---|
| Normal | <120 | and | <80 |
| Elevated | 120−129 | and | <80 |
| Hypertension stage 1 | 130−139 | or | 80−89 |
| Hypertension stage 2 | ≥140 | or | ≥90 |
Adapted from the 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults.31
Figure 2.
Flowchart for the treatment of hypertension.
Adapted from the 7th Brazilian Arterial Hypertension Guideline.32
ACE: angiotensin-converting-enzyme; ARBs: angiotensin II receptor blockers; CCB: calcium channel blockers; CV: cardiovascular.
Diabetes control
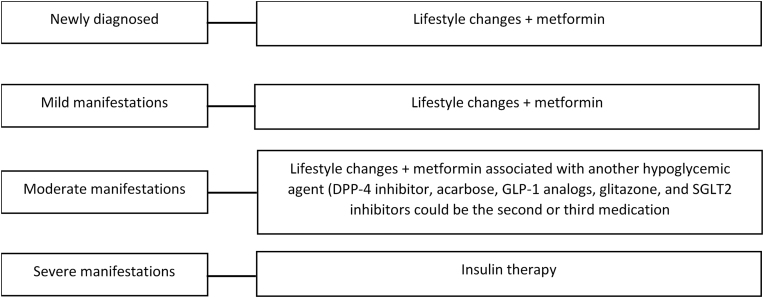
For newly diagnosed patients, initial lifestyle changes, associated with metformin, are recommended (Level of Evidence: A).33 For patients with mild manifestations, when glycemia is less than 200 mg/dL, with mild or absent symptoms, metformin is indicated. In the case of intolerance to metformin, long-acting preparations may be useful. While the problem persists, one of the other hypoglycemic agents may be chosen (Figure 3).33
Figure 3.
Treatment of diabetes mellitus type 2.
Adapted from the Guidelines of the Brazilian Diabetes Society (2015–2016).33
In patients with moderate manifestations, when fasting glycemia is greater than 200 mg/dL, but less than 300 mg/dL in the absence of criteria for severe manifestations, start with lifestyle modifications and metformin associated with another anti-hyperglycemic agent. The indication of the second agent will depend on the predominance of insulin resistance or insulin deficiency/beta cell failure. Thus, the DPP-4 inhibitor, acarbose, GLP-1 analogs, glitazones, and SGLT2 inhibitors could be the second or third medication. In the patient with weight loss, a sulfonylurea or glinides could be combined.33
For patients with glycemic values above 300 mg/dL and severe manifestations (significant weight loss, severe symptoms and/or ketonuria), immediate insulin therapy is recommended.33
Smoking control
Complete smoking cessation is recommended for patients with CML using TKIs. According to the Brazilian Guideline of Dyslipidemia and Atherosclerosis Prevention Update, first-line drugs for smoking cessation are nicotine replacement therapy, with gum or patch, bupropion and varenicline. Some patients can benefit from antitobacco programs and must be referred to them (Grade of Recommendation: I; Level of Evidence: A).29
LDL-c control
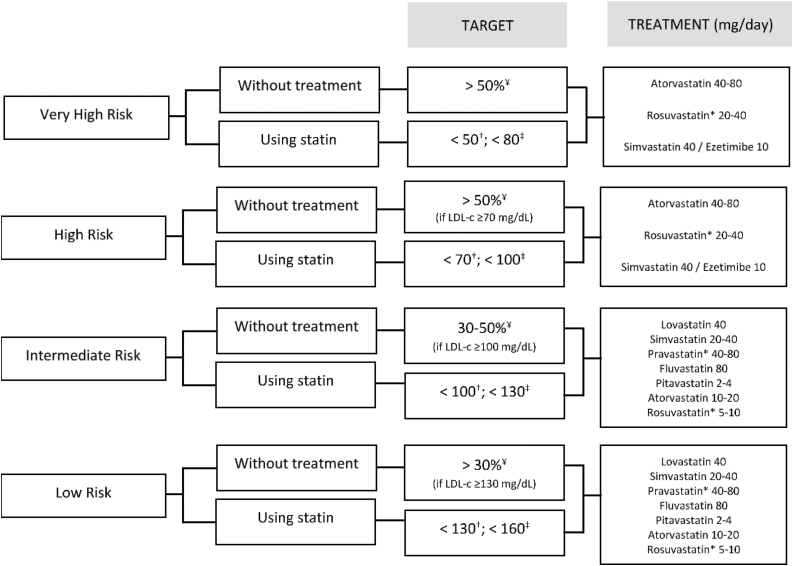
According to the Brazilian Guidelines, LDL-C targets should be guided according to the baseline CV risk of the patient. For very high-risk patients without statin therapy, the goal is to reduce the percentage by 50%. For those on statin therapy, the target is to achieve LDL-c <50 mg/dL or non-HDL-c <80 mg/dL. Treatment with atorvastatin 40–80 mg/day, rosuvastatin 20–40 mg/day or simvastatin 40 mg/day + ezetimibe 10 mg/day is recommended.29
For the high-risk group without statin therapy, the goal is to reduce the percentage by 50%, if LDL-c ≥70 mg/dL. For those on statin therapy, the target is to achieve LDL-c <70 mg/dL or non-HDL-c <100 mg/dL. Treatment with atorvastatin 40–80 mg/day, rosuvastatin 20–40 mg/day or simvastatin 40 mg/day + ezetimibe 10 mg/day is recommended, besides behavioral and dietary measures.29
The intermediate-risk group without statin therapy should reduce the percentage by 30–50%, if LDL-c ≥100 mg/dL. For those on statin therapy, the target is to achieve LDL-c <100 mg/dL or non-HDL-c <130 mg/dL. Treatment with lovastatin 40 mg/day, simvastatin 20–40 mg/day, pravastatin 40–80 mg/day, fluvastatin 80 mg/day, pitavastatin 2−4 mg/day, atorvastatin 10–20 mg/day or rosuvastatin 5–10 mg/day is recommended.29
If classified as low-risk, patients without statin therapy should reduce the percentage by 30%, if LDL-c ≥130 mg/dL. For those on statin therapy, the target is to achieve LDL-c <130 mg/dL or non-HDL-c <160 mg/dL. Treatment with lovastatin 40 mg/day, simvastatin 20–40 mg/day, pravastatin 40–80 mg/day, fluvastatin 80 mg/day, pitavastatin 2–4 mg/day, atorvastatin 10–20 mg/day, rosuvastatin 5–10 mg/day is recommended. Figure 4 presents the target and recommended treatment for the LDL-c control.29
Figure 4.
Targets and recommended treatment for the LDL-c control.
Adapted from the online cardiovascular risk stratification tool developed by the Brazilian Society of Cardiology, Department of Atherosclerosis, Brazilian Society of Endocrinology and Metabology and the Brazilian Society of Diabetes.29
*Rosuvastatin and pravastatin are preferred treatments. Simvastatin and atorvastatin should be used with caution due to the possibility of drug interactions through the metabolism route.
¥Expected % of reduction.
†Target for LDL-c (mg/dL).
‡Target for non-HDL-c (mg/dL).
The rigorous control of LDL-C levels in patients using nilotinib is strongly recommended because of the associated risk of peripheral arterial disease, even in patients with a baseline low CV risk profile.
Monitoring
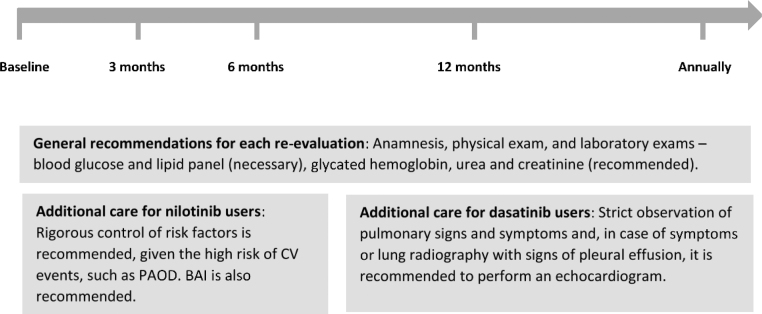
It is important to remember that the CV risk score is dynamic and the control of risk factors can reduce the risk to the patient.29 This expert consensus recommends that the re-evaluation of CV risk after the baseline be performed at 3 months, 6 months and 12 months. After this period, it should be done annually and for specific patients, at the clinician’s discretion. In each re-evaluation, laboratorial exams should be requested (blood glucose, glycated hemoglobin, lipid panel, urea and creatinine). An active and thorough search for symptoms that may suggest complications, such as shortness of breath with dasatinib and leg pain with nilotinib, is strongly recommended.
If the hematologist chooses to start treatment with a first-generation TKI, these general recommendations can be followed. For the second-generation TKIs nilotinib and dasatinib, some specific care should also be taken. Figure 5 presents the main recommendations for monitoring the CV risk during TKI treatment.
Figure 5.
CV risk monitoring during TKI treatment.
BAI: brachial ankle index; CV: cardiovascular; PAOD: peripheral arterial occlusive disease.
Rigorous control of risk factors for CVD in the population using nilotinib and dasatinib is recommended, given the risk of CV complications with these drugs, such as PAOD and pulmonary hypertension. For patients with high or very high CV risk on nilotinib, periodic exams for the evaluation of subclinical atherosclerosis, such as BAI or carotid ultrasonography, are recommended.
For patients using dasatinib, there is a risk of pulmonary hypertension. Considering the severity of this pathology, the strict observation of pulmonary signs and symptoms is recommended and, in the case of symptoms or lung radiography with signs of pleural effusion, an echocardiogram should be performed. There is no consensus in the literature about the optimal periodicity between follow-up echocardiograms. Patients suspected of having pulmonary hypertension related to dasatinib should be referred to a cardiologist for a deeper evaluation and diagnosis confirmation. If dasatinib-induced PAH is confirmed, the treatment should be discontinued.
The rational approach to CV event prophylaxis requires the understanding of the pathophysiology underlying these complications and some decisions need to be individualized. Prophylactic treatments in these patients should be done on an individual evaluation basis. Regarding secondary prevention, when atherosclerotic disease is detected in any territory (even when subclinical), strict control of risk factors and the addition of aspirin and statins are indicated.18 Some restrospective data suggest lower rates of CV events in second generation TKI users on chronic aspirin therapy,34 however, this hypothesis was never assessed in a randomized controlled trial. In the general healthy population, there is a growing body of high-quality evidence driven by big populational trials indicating that, although aspirin use is associated with a lower incidence of CV events, its benefits are largely counterbalanced by higher rates of major bleeding, even in the high baseline CV risk group.35, 36 As a consequence, aspirin is not recommended as a primary prevention strategy in second generation TKI users.
Final considerations
To the best of our knowledge, this is the first Brazilian publication containing recommendations for hematologists concerning the prevention and monitoring of CV aspects specifically in patients with CML. With current treatments, patients with CML have an overall survival resembling that of the general population.4 The evaluation of baseline CV risk and the safe administration of a TKI allow the patients to benefit from the maximum treatment, while avoiding unwanted effects. We believe that these recommendations need to be feasible from the perspective of the Brazilian public and private service centers to achieve good adherence.
New evidence with the long-term follow-up of patients with CML in the use of TKIs is necessary for complete knowledge of the treatment safety.
Funding source
This article was supported in medical writing, funded by Novartis Biociências S.A.
Conflicts of interest
This article was supported in medical writing, funded by Novartis Biociências S.A.
Acknowledgements
We thank the SENSE Company Brazil for editorial support in developing drafts of this manuscript. The authors were responsible for critical revisions of the manuscript for important intellectual content. The editorial support was funded by Novartis Brasil.
References
- 1.Sociedade Brasileira de Oncologia Clínica (SBOC) Manual de Condutas 2011. Rev Soc Bras Oncol Clin. 2011:589. [Google Scholar]
- 2.Chen Y., Wang H., Kantarjian H., Cortes J. Trends in chronic myeloid leukemia incidence and survival in the United States from 1975 to 2009. Leuk Lymphoma. 2013;54(7):1411–1417. doi: 10.3109/10428194.2012.745525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministério da Saúde (Brasil) Ministério da Saúde; Brasília: 2014. Secretaria de Atenção à Saúde. Protocolos Clínicos e Diretrizes Terapêuticas em Oncologia. [Google Scholar]
- 4.Bower H., Björkholm M., Dickman P.W., Höglund M., Lambert P.C., Andersson T.M.L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851–2857. doi: 10.1200/JCO.2015.66.2866. [DOI] [PubMed] [Google Scholar]
- 5.Breccia M., Arboscello E., Bellodi A., Colafigli G., Molica M., Bergamaschi M. Proposal for a tailored stratification at baseline and monitoring of cardiovascular effects during follow-up in chronic phase chronic myeloid leukemia patients treated with nilotinib frontline. Crit Rev Oncol Hematol. 2016;107:190–198. doi: 10.1016/j.critrevonc.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Goldman J.M., Melo J.V. Chronic Myeloid Leukemia — advances in biology and new approaches to treatment. N Engl J Med. 2003;349(15):1451–1464. doi: 10.1056/NEJMra020777. [DOI] [PubMed] [Google Scholar]
- 7.Deininger M., O’Brien S.G., Guilhot F., Goldman J.M., Hochhaus A., Hughes T.P. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood. 2009;114 Abstract1126. [Google Scholar]
- 8.Giles F.J., le Coutre P.D., Pinilla-Ibarz J., Larson R.A., Gattermann N., Ottmann O.G. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27(1):107–112. doi: 10.1038/leu.2012.181. [DOI] [PubMed] [Google Scholar]
- 9.Shah N.P., Kim D.W., Kantarjian H., Rousselot P., Llacer P.E., Enrico A. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95(2):232–240. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larson R.A., Hochhaus A., Hughes T.P., Clark R.E., Etienne G., Kim D.W. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H.M., Shah N.P., Cortes J.E., Baccarani M., Agarwal M.B., Undurraga M.S. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119(5):1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalmanti L., Saussele S., Lauseker M., Müller M.C., Dietz C.T., Heinrich L. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia. 2015;29(5):1123–1132. doi: 10.1038/leu.2015.36. [DOI] [PubMed] [Google Scholar]
- 13.Gambacorti-Passerini C., Kantarjian H.M., Kim D.W., Khoury H.J., Turkina A.G., Brümmendorf T.H. Long-term efficacy and safety of bosutinib in patients with advanced leukemia following resistance/intolerance to imatinib and other tyrosine kinase inhibitors. Am J Hematol. 2015;90(9):755–768. doi: 10.1002/ajh.24034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes J.E., Kantarjian H., Shah N.P., Bixby D., Mauro M.J., Flinn I. Ponatinib in refractory philadelphia chromosome–positive leukemias. N Engl J Med. 2012;367(22):2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krause D.S., Van Etten R.A. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353(2):172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 16.Bellinger A.M., Arteaga C.L., Force T., Humphreys B.D., Demetri G.D., Druker B.J. Cardio-oncology: how new targeted cancer therapies and precision medicine can inform cardiovascular discovery. Circulation. 2015;132(23):2248–2258. doi: 10.1161/CIRCULATIONAHA.115.010484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aghel N., Delgado D.H., Lipton J.H. Cardiovascular toxicities of BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: preventive strategies and cardiovascular surveillance. Vasc Health Risk Manag. 2017;13:293–303. doi: 10.2147/VHRM.S108874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moslehi J.J., Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33(35):4210–4218. doi: 10.1200/JCO.2015.62.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rix U., Hantschel O., Dürnberger G., Remsing Rix L.L., Planyavsky M., Fernbach N.V. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110(12):4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 20.Douxfils J., Haguet H., Mullier F., Chatelain C., Graux C., Dogné J.M. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival. JAMA Oncol. 2016;2(5):625–632. doi: 10.1001/jamaoncol.2015.5932. [DOI] [PubMed] [Google Scholar]
- 21.Dahlén T., Edgren G., Lambe M., Höglund M., Björkholm M., Sandin F. Cardiovascular events associated with use of tyrosine kinase inhibitors in chronic myeloid leukemia. Ann Intern Med. 2016;165(3):161–166. doi: 10.7326/M15-2306. [DOI] [PubMed] [Google Scholar]
- 22.Le Coutre P., Rea D., Abruzzese E., Dombret H., Trawinska M.M., Herndlhofer S. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst. 2011;103(17):1347–1348. doi: 10.1093/jnci/djr292. [DOI] [PubMed] [Google Scholar]
- 23.Giles F.J., Mauro M.J., Hong F., Ortmann C.E., McNeill C., Woodman R.C. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia. 2013;27(6):1310–1315. doi: 10.1038/leu.2013.69. [DOI] [PubMed] [Google Scholar]
- 24.Hochhaus A., Saglio G., Hughes T.P., Larson R.A., Kim D.W., Issaragrisil S. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes J.E., Saglio G., Kantarjian H.M., Baccarani M., Mayer J., Boqué C. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34(20):2333–2340. doi: 10.1200/JCO.2015.64.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carneiro B.A., Kaplan J.B., Giles F.J. Tyrosine kinase inhibitor therapy in chronic myeloid leukemia: update on key adverse events. Expert Rev Hematol. 2015;8(4):457–479. doi: 10.1586/17474086.2015.1041910. [DOI] [PubMed] [Google Scholar]
- 27.Coutinho A.D., Makenbaeva D., Farrelly E., Landsman-Blumberg P.B., Lenihan D. Elevated cardiovascular disease risk in patients with chronic myelogenous leukemia seen in community-based oncology practices in the United States. Clin Lymphoma Myeloma Leuk. 2017;17(10):676–683. doi: 10.1016/j.clml.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Assunção P.M., Lana T.P., Delamain M.T., Duarte G.O., Zulli R., Lorand-Metze I. Cardiovascular risk and cardiovascular events in patients with chronic myeloid leukemia treated with tyrosine kinase-inhibitors. Clin Lymphoma Myeloma Leuk. 2019;19(3):162–166. doi: 10.1016/j.clml.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Faludi A., Izar M., Saraiva J., Chacra A.P.M., Bianco H.T., Neto Afiune A. Atualização da diretriz brasileira de dislipidemias e prevenção da aterosclerose - 2017. Arq Bras Cardiol. 2017;109(1):1–76. doi: 10.5935/abc.20170121. [DOI] [PubMed] [Google Scholar]
- 30.D’Agostino R.B., Vasan R.S., Pencina M.J., Wolf P.A., Cobain M., Massaro J.M. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 31.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr, Collins K.J., Dennison Himmelfarb C. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J Am Coll Cardiol. 2017;71(19):2199–2269. [Google Scholar]
- 32.Malachias M.V.B., WKSB Souza, Plavnik F.L., Rodrigues C.I.S., Brandão A.A., Neves M.F.T. 7th Brazilian guideline of arterial hypertension. Arq Bras Cardiol. 2016;107(3):1–103. doi: 10.5935/abc.20160151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sociedade Brasileira de Diabetes . A.C. Farmacêutica; São Paulo: 2016. Diretrizes da Sociedade Brasileira de Diabetes 2015-2016. Available from: www.diabetes.org.br/sbdonline/images/docs/DIRETRIZES-SBD-2015-2016.pdf [cited 07.03.18] [Google Scholar]
- 34.Caocci G., Mulas O., Annunziata M., Luciano L., Bonifacio M., Orlandi E.M. Cardiovascular toxicity in patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors in the real-life practice: identification of risk factors and the role of prophylaxis. Am J Hematol. 2018;93(7):E159–61. doi: 10.1002/ajh.25102. [DOI] [PubMed] [Google Scholar]
- 35.The ASCEND Study Collaborative Group Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529–1539. doi: 10.1056/NEJMoa1804988. [DOI] [PubMed] [Google Scholar]
- 36.McNeil J.J., Woods R.L., Nelson M.R., Reid C.M., Kirpach B., Wolfe R. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379(16):1499–1508. doi: 10.1056/NEJMoa1800722. [DOI] [PMC free article] [PubMed] [Google Scholar]