Figure 5.
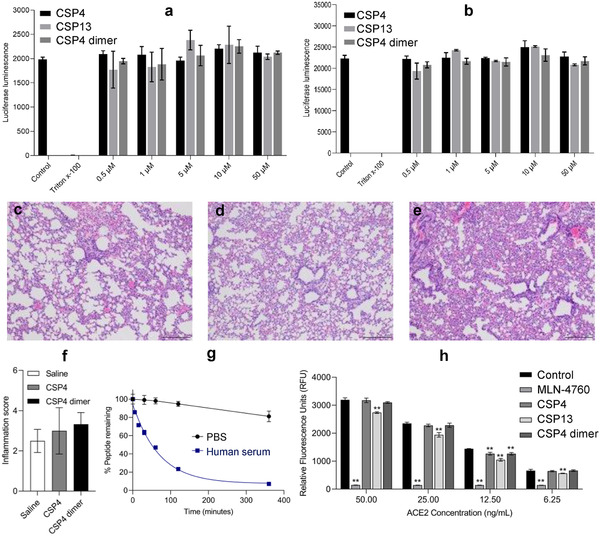
Safety and stability evaluation of the anti‐ACE2 peptides. a) Cytotoxicity of CSP4, CSP13, and CSP4 dimer peptides in Vero‐E6 cells at different concentrations. Triton X‐100 was used as a positive control. b) Cytotoxicity of CSP4, CSP13, and CSP4 dimer peptides in Huh‐7 cells at different concentrations. Triton X‐100 was used as a positive control. Representative H&E staining of lung specimens from the mice (3–4 mice per group) 72 h after intratracheal administration of c) saline, d) CSP4, and e) CSP4 dimer peptides (Scale bar: 100 µm). The peptides were administrated at a dose of 2 mg kg−1. f) Inflammation scores (0–5 scale) of the lung specimens. g) Stability of CSP4 dimer peptide in PBS and human serum. h) Effect of anti‐ACE2 peptides on ACE2 enzyme activity. The ACE2 inhibitor MLN‐4760 was used as a positive control. Cytotoxicity and ACE2 enzyme activity results are presented as the mean ± SD (n = 3). Statistical significance was determined by one‐way ANOVA (a, b, f, and h) with Tukey's multiple comparison. ** p < 0.01.
