Abstract
Objective
Continuation and maintenance ECT (c‐/m‐ECT) are effective in the prevention of relapse and recurrence of both affective and psychotic disorders. However, data are scarce concerning the trajectories of severe mental disorders after the end of c‐/m‐ECT. This prospective study investigates the clinical outcome of patients with versus without modifications of their c‐/m‐ECT schedules.
Methods
In the context of the COVID‐19 pandemic, ECT capacities were restricted at many clinics in early 2020. All patients receiving c‐/m‐ECT in March and April 2020 at our department (n = 53, unipolar depression, bipolar disorder, schizophrenia) were followed up for six months to investigate the impact of treatment modifications imposed by the pandemic. Based on individual decisions, c‐/m‐ECT was either (a) continued without modification, (b) continued with reduced frequency, or (c) discontinued.
Results
Both reduced frequency and discontinuation of c‐/m‐ECT were associated with significant clinical deterioration as measured by CGI‐I (Clinical Global Impression Scale ‐ Global Improvement) during the six‐month follow‐up when compared to the subgroup of patients without any treatment modification (p = 0.005, p = 0.011). Furthermore, patients with discontinued or reduced c‐/m‐ECT showed significantly higher rates of rehospitalizations (p = 0.028) and new acute courses of ECT (p = 0.018).
Conclusion
Despite the limitations of a heterogeneous and relatively small sample, our study strongly corroborates the effectiveness of c‐/m‐ECT in a real‐world population. Especially, patients with shorter time since index ECT seem to be at high risk for severe clinical deterioration in the case of treatment discontinuation or reduction.
Keywords: electroconvulsive therapy, maintenance ECT, continuation ECT, COVID‐19, effectiveness
Significant Outcomes
In a mixed sample of patients receiving maintenance ECT (mECT), both discontinuation and reduced frequency of the treatment were associated with significant clinical deterioration.
Especially patients with shorter time since index ECT seem to be at high risk for relapse.
Our data support the effectiveness of mECT in a real‐world clinical sample.
Limitations
The naturalistic, observational design of our study and the heterogeneous sample limit causal conclusions and the deeper analysis of potential confounders.
Patients with more severe symptoms or more relapses in the past were more likely to continue with mECT according to our clinical decision, which may have influenced the outcome.
1. INTRODUCTION
The efficacy of electroconvulsive therapy (ECT) as an acute treatment for depressive, psychotic, and manic episodes has been empirically validated in numerous studies. 1 , 2 , 3 Despite its effectiveness as an acute treatment, a meta‐analysis has shown high relapse rates of approximately 50% in a one‐year follow‐up in patients with depressive disorders. Here, the highest risk of relapse occurred during the first six months after index treatment. 4 Therefore, different therapeutic strategies have been established to reduce the risk of an early relapse. Specifically, continuation and maintenance ECT are provided after the index treatment to prevent relapse and recurrence.
A growing body of literature supports the effectiveness of c‐/m‐ECT in combination with pharmacotherapy in the prevention of relapse and recurrence of both affective 5 , 6 and psychotic 7 , 8 disorders. An overview of clinical practice recommendations for c‐/m‐ECT including concomitant medication, electrode placement, and stimulus dose was published 2018 by Gill and Kellner based on a workshop outcome. 9
Different strategies of c‐/m‐ECT have been described and their effectiveness proven with study periods ranging from six to 12 months. For example, the PRIDE study showed a high effectiveness of a flexible treatment algorithm of continuation ECT (following four fixed treatments in the first month) based on the individual's psychopathology. 10 In the study by Nordenskjöld et al., patients were randomized to receive either pharmacotherapy and a fixed c‐/m‐ECT algorithm (weekly ECT for six weeks, followed by biweekly ECT for 46 more weeks) or pharmacotherapy only. 32% of the patients treated with c‐/m‐ECT and pharmacotherapy relapsed compared to 61% of the patients treated with pharmacotherapy only. 11 Scientific evidence regarding the effectiveness of c‐/m‐ECT for more than one year after the index ECT series rarely exists.
Furthermore, there is hardly any evidence regarding the consequences of discontinuation and reduced frequency of c‐/m‐ECT. To our knowledge, only two recent studies 12 , 13 provide data on this topic. Martinez‐Amoros et al. retrospectively analyzed 73 patients with depression, bipolar disorder, and schizophrenia for at least one year after discontinuation of c‐/m‐ECT. After stopping c‐/m‐ECT, 49.3% of the patients relapsed. Here, a shorter interval between the sessions before discontinuation of c‐/m‐ECT as well as more previous episodes of illness were associated with a higher risk of relapse. 13 In their retrospective analysis with varying individual follow‐up intervals, Cabelguen et al. found that nine out of 18 patients with unipolar, bipolar, or schizoaffective disorder relapsed after discontinuation of c‐/m‐ECT, with 44% of the relapses occurring during the first six months after treatment cessation. 12
1.1. Aims of the study
The COVID‐19 pandemic with the consequence of reduced treatment capacities now provides an opportunity to investigate the impact of a reduced frequency or even complete discontinuation of c‐/m‐ECT on the disease trajectories in a naturalistic, real‐world sample of 53 patients. To our knowledge, our study is the first to prospectively examine disease progression after discontinuation of c‐/m‐ECT.
2. MATERIAL AND METHODS
2.1. Study design
2.1.1. Modification of c‐/m‐ECT because of the COVID‐19 pandemic
COVID‐19 (SARS‐CoV‐2) had a great impact on the healthcare system. In March 2020, all elective procedures at the University Medical Center Göttingen including the Department of Psychiatry and Psychotherapy were stopped. Although ECT and also c‐/m‐ECT by no means are elective treatments, 14 the ECT program also had to be markedly reduced mostly because of limited anesthesia capacities. Consequently, there was an abrupt reduction of the frequency or even a complete discontinuation of most c‐/m‐ECT treatments. In absence of any evidence‐based methods, 15 we triaged our patients on an individual basis taking into account their current severity of illness and the estimated likelihood of relapse. The attending psychiatrist together with the patient or caregiver decided whether c‐/m‐ECT was (1) continued without modification, (2) continued with reduced frequency or (3) stopped.
2.1.2. Follow‐up for 6 months
All c‐/m‐ECT patients were followed up for at least six months until October 2020 to evaluate the consequences of treatment modification. Patients and/or caregivers were interviewed on‐site or—because of the pandemic—mostly by telephone regarding their well‐being. Clinical outcome was measured using the Clinical Global Impression Scale ‐ Global Improvement (CGI‐I) as primary outcome. 16 The CGI‐I allows the rater to assess the change of the patient's condition compared to the time before an intervention on a seven‐point rating scale from 1 = “very much improved” to 7 = “very much worse.” In addition, most patients were treated in our outpatient department so that medical files could be analyzed. Besides CGI‐I, rehospitalizations and new acute ECT treatments were documented and rated as surrogates for clinical deterioration.
2.2. Sample
In March/April 2020, 53 patients received c‐/m‐ECT at University Medical Center Göttingen, Department of Psychiatry and Psychotherapy. The patients were aged between 16 and 82 (M = 59,58, SD =13,07). Of the total sample, 58.8% (n = 31) were female. Most patients were diagnosed with unipolar depressive disorder (n = 26; ICD‐10: F32.1 to F32.3 and F33.1 to F33.3), bipolar disorder (n = 9; ICD‐10: F31.3 to F31.5), and schizophrenia (n = 8, ICD‐10: F20.x; see Table 1 for details). The intervals of c‐/m‐ECT treatments largely varied between the individual patients with a range between one and twelve weeks. Please see Table 1 for detailed patient characteristics.
TABLE 1.
Patient characteristics
| Variable | Whole sample (N = 53) | c‐/m‐ECT without modification (n = 7) | c‐/m‐ECT frequency reduced (n = 12) | c‐/m‐ECT discontinuation (n = 34) |
|---|---|---|---|---|
| Age | M = 59.58 ± 13.07 | M = 50.71 ± 17.14 | M = 61.83 ± 11.08 | M = 60.62 ± 12.46 |
| Gender (male; female) | 22 (41.5%); 31 (58.5%) | 0 (0.0%); 7 (100.0%) | 5 (41.7%); 7 (58.3%) | 17 (50.0%); 17 (50.0%) |
| ECT: Electrode placement | ||||
| Right unilateral | n = 16 (30.2%) | n = 1 (14.3%) | n = 5 (41.7%) | n = 10 (29.4%) |
| Left anterior, right temporal | n = 19 (35.8%) | n = 4 (57.1%) | n = 5 (41.7%) | n = 10 (29.4%) |
| Bitemporal | n = 18 (34.0%) | n = 2 (28.6%) | n = 2 (16.7%) | n = 14 (41.2%) |
| c‐/m‐ECT characteristics | ||||
| Interval before modification (weeks) | Mdn = 3 (IQR = 3) | Mdn = 1 (IQR = 5) | Mdn = 1.5 (IQR = 1) | Mdn = 4 (IQR = 4) |
| Interval unchanged since (weeks) | Mdn = 8 (IQR = 3) | Mdn = 18 (IQR = 25) | Mdn = 2 (IQR = 7) | Mdn = 9 (IQR = 10) |
| Time since index ECT (months) | Mdn = 4 (IQR = 11) | Mdn = 7 (IQR = 8) | Mdn = 0.5 (IQR = 2) | Mdn = 8.5 (IQR = 11) |
| Diagnoses (ICD−10) | ||||
| Unipolar depression (F32/F33) | n = 26 (49.1%) | n = 4 (57.1%) | n = 6 (50.0%) | n = 16 (47.1%) |
| Bipolar disorder (F31) | n = 9 (17.0%) | n = 1 (14.3%) | n = 2 (16.7%) | n = 6 (17.6%) |
| Schizophrenia (F20) | n = 8 (15.1%) | n = 1 (14.3%) | n = 1 (8.3%) | n = 6 (17.6%) |
| Other a | n = 10 (18.9%) | n = 1 (14.3%) | n = 3 (25.0%) | n = 6 (17.6%) |
| Antidepressant | ||||
| SSRI | n = 8 (15.1%) | n = 1 (14.3%) | n = 2 (16.7%) | n = 5 (14.7%) |
| SNRI | n = 15 (28.3%) | n = 3 (42.9%) | n = 3 (25.0%) | n = 9 (26.5%) |
| Tricyclic | n = 3 (5.7%) | n = 1 (14.3%) | n = 0 (0.0%) | n = 2 (5.9%) |
| MAO‐I | n = 2 (3.8%) | n = 0 (0.0%) | n = 0 (0.0%) | n = 2 (5.9%) |
| Mirtazapine | n = 1 (1.9%) | n = 0 (0.0%) | n = 1 (8.3%) | n = 0 (0.0%) |
| Other | n = 2 (3.8%) | n = 1 (14.3%) | n = 1 (8.3%) | n = 0 (0.0%) |
| Combination | n = 6 (11.3%) | n = 1 14.3 (%) | n = 2 (16.7%) | n = 3 (8.8%) |
| None | n = 16 (30.2%) | n = 0 (0.0%) | n = 3 (25.0%) | n = 13 (38.2%) |
| Antipsychotic | ||||
| Atypical | n = 27 (50.9%) | n = 4 (57.1%) | n = 6 (50.0%) | n = 17 (50.0%) |
| Low‐potency | n = 1 (1.9%) | n = 0 (0.0%) | n = 0 (0.0%) | n = 1 (2.9%) |
| Combination | n = 12 (22.6%) | n = 1 (14.3%) | n = 4 (33.3%) | n = 7 (20.6%) |
| None | n = 13 (24.5%) | n = 2 (28.6%) | n = 2 (16.7%) | n = 9 (26.5%) |
| Mood stabilizer | ||||
| Lithium | n = 15 (%) | n = 3 (42.9%) | n = 2 (16.7%) | n = 10 (29.4%) |
| Other | n = 6 (%) | n = 0 (0.0%) | n = 2 (16.7%) | n = 4 (11.8%) |
| None | n = 32 (%) | n = 4 (57.1%) | n = 8 (66.7%) | n = 20 (58.8%) |
Captions: M = mean ±standard deviation; Mdn = median; IQR = interquartile range.
Other included F25.x (n = 5), F06.x (n = 4), F44.4 (n = 1).
2.3. Statistical analysis
IBM SPSS Statistics 26 (IBM Corp. Armonk, NY) was used for data analysis. For numeric variables, means (M) and standard deviations (SD) were computed. As some variables with regard to the initial c‐/m‐ECT characteristics (interval before modification, interval unchanged since, time since index ECT) were heterogeneously distributed in our sample, medians (Mdn) with interquartile ranges (IQR) were computed for descriptive statistics (see Table 1 ).
We used an UNIANOVA to analyze differences for our primary outcome (CGI‐I) between the three subgroups based upon the modification of c‐/m‐ECT: (1) continuation without modification, (2) continuation with reduced frequency, (3) discontinuation. For multiple comparisons within the model, p‐values were corrected using the Bonferroni method (initial significance: p < 0.05, two‐tailed). In addition, we did an analysis for the secondary outcomes: Both binary variables measuring illness aggravation (yes vs. no: (1) rehospitalization; (2) new acute ECT series) were analyzed for differences between the three subgroups (2×3 matrices). For this purpose, the Freeman‐Halton extension of Fisher's exact test was used, 17 as sample sizes fell below n = 5 in multiple cells (see results section). An additional exploratory analysis was conducted to identify predictors for relapse/recurrence—please see results section and Table 2 for further details.
TABLE 2.
Exploratory analysis: Predictors for relapse/recurrence in patients with modification of c‐/m‐ECT
| Variable | Clinical deterioration measured by: | |||||
|---|---|---|---|---|---|---|
| CGI‐I score (≤4 vs. ≥5) | p | Rehospitalization or new ECT (no vs. yes) | p | |||
| ≤ 4 (n = 19) | ≥ 5 (n = 27) | no (n = 22) | yes (n = 24) | |||
| Age | M = 61.11 ± 12.64 | M = 60.81 ± 11.79 | 0.937 | M = 61.64 ± 11.94 | M = 60.29 ± 12.29 | 0.709 |
| Gender (male; female) | 8 (42.1%); 11 (57.9%) | 14 (51.9%); 13 (48.1%) | 0.515 | 11 (50.0%); 11 (50.0%) | 11 (45.8%); 13 (54.2%) | 0.777 |
| c‐/m‐ECT characteristics | ||||||
| Interval before modification (weeks) | Mdn =4 (IQR =5) | Mdn =2 (IQR =3) | 0.002 ** | Mdn =4 (IQR =4) | Mdn =2 (IQR =3) | 0.003 ** |
| Interval unchanged since (weeks) | Mdn =10 (IQR =9) | Mdn =6 (IQR =8) | 0.263 | Mdn =10.5 (IQR =12) | Mdn =6 (IQR =6) | 0.158 |
| Time since index ECT (months) | Mdn =9 (IQR =13) | Mdn =2 (IQR =10) | 0.018 * | Mdn =10 (IQR =13) | Mdn =2 (IQR =8) | 0.010 ** |
*p < 0.01, **p < 0.01. Captions: M = mean ±standard deviation; Mdn =median; IQR =interquartile range; uncorrected p‐values for age (t‐tests), gender (2×2 χ2‐tests) and c‐/m‐ECT characteristics (Mann‐Whitney‐U‐tests).
2.4. Ethical approval
This study has been approved by the local ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
3. RESULTS
3.1. Modification of c‐/m‐ECT due to the COVID‐19 pandemic
Based on the shared decision‐making between the attending psychiatrist and the patient or caregiver, c‐/m‐ECT was (1) continued without modification in n = 7 (13.2%) patients, (2) reduced in n = 12 (22.6%) patients with the interval between the sessions averagely doubled from initial M = 1.75 weeks to M = 3.50 weeks, and (3) discontinued for the majority of n = 34 (64.2%) patients. For details regarding the distribution of diagnoses and treatment characteristics within the subgroups, please see Table 1.
Reasons for the unmodified continuation of c‐/m‐ECT in n = 7 patient were the persistence of marked residual symptoms (6 out of 7), lack of treatment alternatives (4 out of 7) and patients’ explicit wish (4 out of 7). Criteria leading to either modification or discontinuation of c‐/m‐ECT were a rather stable psychopathology in terms of response/remission (39 out of 46), and patients at risk of a more serious COVID‐19 infection in the healthcare setting because of higher age or severe medical condition (15 out of 46). Only a minority of patients explicitly asked for a modification themselves (7 out of 46), mostly in the context of self‐reported cognitive deficits (7 out of 46) and restrictions by their assisted living facility during the pandemic (eg, need for isolation after hospital stay; 3 out of 46).
3.1.1. Effects of c‐/m‐ECT‐modification
Throughout the six months follow‐up, we assessed the disease trajectories in the following three subgroups: (1) continuation without modification, (2) continuation with reduced frequency, and (3) discontinuation.
3.1.2. Primary outcome: CGI‐I
The UNIANOVA revealed significant differences in the CGI‐I between the three subgroups (F(2, 50) = 5.98, p = 0.005; see Figure 1): Patients without any modification showed the lowest CGI‐I score (M = 3.57, SD = 0.98) meaning “no change” or even “minimal improvement.” The groups with reduced frequency of c‐/m‐ECT (M = 5,50, SD = 1,00) and discontinued c‐/m‐ECT (M = 5.12, SD= 1.32) showed higher CGI‐I scores, meaning “minimally worse” or even “much worse.” Bonferroni corrected pairwise comparisons revealed significant differences between the subgroup without modification of c‐/m‐ECT and both groups with reduced frequency (p = 0.005) as well as discontinuation (p = 0.011). There was no significant difference between the groups with reduced frequency and discontinuation (p = 1.00).
FIGURE 1.
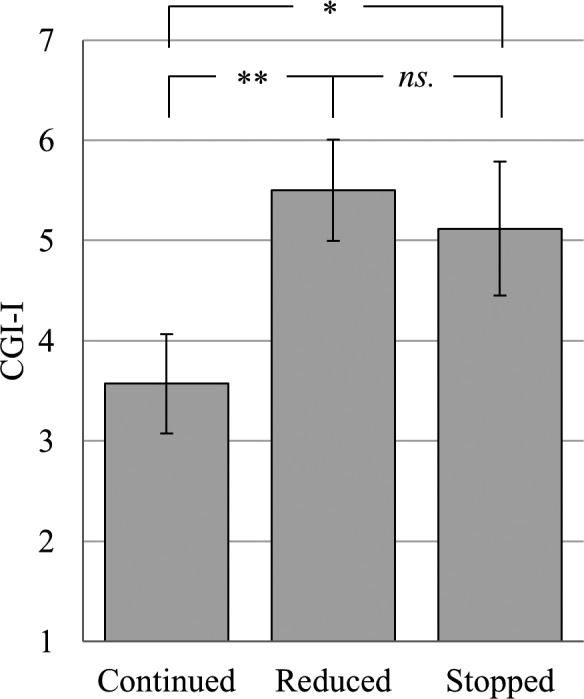
Differences in the CGI‐I between the three subgroups. Mean values with 95%‐CIs and Bonferroni corrected pairwise comparisons of the patients’ CGI‐I score (range from 1 = “very much improved” to 7 = “very much worse”) for the c‐/m‐ECT subgroups: (1) continuation without modification (n = 7), (2) reduced frequency (n = 12), (3) discontinuation (n = 34), *p < 0.05, **p < 0.01
3.1.3. Secondary outcomes: Indicators of clinical deterioration
Overall, n = 25 (47.2%) patients showed a clinical deterioration in terms of rehospitalization during the six months follow‐up. In the different subgroups, rehospitalization rates were n = 1 out of 7 (14.3%) for the group without modification of c‐/m‐ECT, compared to n = 9 out of 12 (75.0%) for the group with reduced c‐/m‐ECT frequency, and n = 15 out of 34 (44.1%) for the group with c‐/m‐ECT discontinuation. These observed frequencies differed significantly from the expected frequencies (p = 0.028). Furthermore, n = 15 (28.3%) patients of the total sample received a new acute course of ECT. This indication of deterioration was again least frequent for the subgroup without modification of c‐/m‐ECT (n = 0, 0.0%) compared to patients with reduced frequency (n = 7, 58.3%) or discontinuation of c‐/m‐ECT (n = 8, 23.5%). Again, these observed frequencies differed significantly from the expected frequencies (p = 0.018).
3.1.4. Exploratory analysis: Predictors for relapse/recurrence
To identify factors that possibly mediated the risk of relapse/recurrence after reduction or discontinuation of c‐/m‐ECT, we conducted an exploratory analysis for the group of patients that were subject to any modification of c‐/m‐ECT (n = 46; ie, reduced frequency or discontinuation). This group was then divided with respect to the occurrence of a clinical deterioration as measured by two different discriminator variables, for separate analysis: (1) CGI‐I scores (≥ 5 vs. ≤4), (b) rehospitalization and/or new acute course of ECT (yes vs. no). These groups were then compared with respect to differences in baseline characteristics (gender, age, c‐/m‐ECT interval, period of unchanged c‐/m‐ECT interval, time since index ECT; please see Table 2 for details).
While there were no significant differences between patients with clinical deterioration and their respective comparison groups regarding age and gender (p from 0.515 to 0.937), two out of three baseline characteristics of c‐/m‐ECT differed significantly between the subgroups for both discriminator variables. Patients with clinical deterioration had shorter c‐/m‐ECT intervals at baseline (p from 0.002 to 0.003) and a significantly shorter time since index ECT (p from 0.010 to 0.018; see Table 2 for details) when compared to the group without clinical deterioration.
4. DISCUSSION
In our prospective, naturalistic study, patients with reduced frequency or complete discontinuation of c‐/m‐ECT showed a significant clinical deterioration as measured with CGI‐I during the six months follow‐up in comparison to patients with unchanged c‐/m‐ECT schedules. In addition, patients with reduced and discontinued treatment showed significantly higher rates of both rehospitalization and new acute ECT series within the first six months after cessation of c‐/m‐ECT. In total, 27 out of 46 patients (58.7%) who were affected by any change in their c‐/m‐ECT experienced some kind of clinical deterioration (ie, CGI‐I ≥ 5 and/or rehospitalization and/or new acute ECT). In contrast, only one out of seven patients without treatment modification met one of these criteria. This difference is even more interesting, as the patients without modification of c‐/m‐ECT had the highest risk for relapse according to our clinical judgement at baseline.
To the best of our knowledge, this is the first prospective study investigating the course of severe psychiatric disorders after modification or discontinuation of c‐/m‐ECT and the first study on this topic with an, albeit small, control group. All patients who were treated with c‐/m‐ECT in March/April 2020 at our department were included and followed up for the same six‐month period. Thus, the results of our real‐world sample should allow for inferences for routine clinical practice.
Our findings are largely in line with the results of previous retrospective studies regarding the course of severe psychiatric disorders after discontinuation of c‐/m‐ECT. Martinez‐Amorós et al. 13 investigated the disease trajectories of 73 patients with unipolar depression, bipolar disorder, and schizophrenia who were followed up for at least one year. Relapse/recurrence was defined as required hospitalization or new ECT series. 49.3% of the patients relapsed with a mean time to relapse of 13.39 months after treatment discontinuation. The retrospective analysis by Cabelguen et al. 12 comprised 18 patients with unipolar depression, bipolar disorder, and schizoaffective disorder with individual follow‐up periods ranging from seven to 62 months. Nine of these patients (= 50%) relapsed and four relapses occurred within the first six months after treatment discontinuation. Huuhka et al. 18 also retrospectively examined a mixed sample of 45 patients (diagnosed with unipolar depression, bipolar disorder, schizophrenia, and schizoaffective disorder) one year after discontinuation of c‐/m‐ECT and found a relapse rate of 44%. In the majority of patients, the relapse (defined as hospitalization or new ECT series) occurred during the first three months after the end of the treatment. In contrast to these findings, an earlier study investigated 19 patients with major depressive disorder with a median c‐/m‐ECT period of 26 months and concluded that the reduction of hospitalizations achieved during the period of c‐/m‐ECT was maintained after discontinuation of c‐/m‐ECT. 19 However, conclusions from this study are limited because of methodological issues with regard to data collection and marked heterogeneity of the follow‐up interval.
In our study, we identified possible risk factors for a relapse after modification or discontinuation of c‐/m‐ECT. The patients with clinical deterioration were characterized by a significantly shorter time since index ECT at baseline. Consistent with this, previous studies have shown that a lack of continuation treatment following an acute series of ECT as well as a short time of continuation treatment is associated with a higher risk of relapse. 4 , 5
Furthermore, patients with clinical deterioration had significantly shorter intervals between the c‐/m‐ECT sessions at baseline. Similar results were found in the sample examined by Martínez‐Amorós et al., 13 where patients with intervals of less than four weeks had an increased risk of relapse. This might suggest that patients with longer intervals between the c‐/m‐ECT sessions might better tolerate a discontinuation of ECT. However, this finding may be biased for different reasons. First, patients with short c‐/m‐ECT intervals most likely also have a shorter time since index ECT, which seems to be a risk factor per se (see above). Second, patients with longer c‐/m‐ECT intervals probably have a more stable psychopathology than those with shorter intervals and thus better tolerate a short‐term treatment pause. Nevertheless, they may experience a relapse after longer periods without ECT. Regarding the results of the earlier studies that still found a substantial number of relapses after more than six months of treatment discontinuation, 12 , 13 a longer follow‐up in our study could have led to even more cases with deterioration.
4.1. Limitations
There are some limitations regarding this study. First, our participants represent a quite typical real‐world sample of patients receiving c‐/m‐ECT. Because of this naturalistic design, our sample is heterogeneous regarding the included diagnoses and patients’ concomitant medication. The even smaller sample size of the subgroups did not allow for a more detailed analysis of possibly differential effects of diagnostic subgroups or specific pharmacological treatments 20 on the disease trajectories. Second, because of the abrupt reduction of treatment capacities in the context of the COVID‐19 pandemic, only a minority of patients were able to proceed with their regular ECT schedules. The validity of our results is thus restricted as only a small number of patients without modification of c‐/m‐ECT could be studied. Third, modification of c‐/m‐ECT was not randomized for obvious reasons. According to our clinical judgement at baseline, patients without modification of c‐/m‐ECT had the highest risk for relapse, and it cannot be ruled out that the criteria we used to make the decision regarding the continuation, modification, or discontinuation of c‐/m‐ECT themselves had an impact on the study outcome. Overall, no causal conclusions should be drawn from the results presented here. Nevertheless, they strongly corroborate the effectiveness of c‐/m‐ECT in a naturalistic, real‐world sample. Lastly, our study has no prospective design in the strictest sense. As the changes imposed by the COVID‐19 pandemic had to be implemented very quickly, we were not able to perform a more in‐depth clinical characterization of our patients (e.g. by using diagnosis‐specific rating scales) at study entry. There were no fixed visits in scheduled intervals. Nevertheless, all patients were continuously followed up throughout the six months period either as patients in our outpatient department or, alternatively, via on‐site or telephone interviews. This approach allowed for an uninterrupted follow‐up of all patients in a quasi‐prospective manner.
Despite the limitations of a heterogeneous sample and relatively small subsamples, our study strongly corroborates the effectiveness of c‐/m‐ECT in a real‐world population. Both treatment discontinuation and reduced frequency of c‐/m‐ECT may negatively affect the course of severe affective and psychotic disorders. Especially, patients with shorter time since index ECT and those with short intervals between the c‐/m‐ECT sessions seem to be at high risk for severe clinical deterioration during the first six months after treatment modification. In the absence of data from randomized controlled trials, the findings of our study may support clinical decision‐making in situations of limited ECT capacities.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/acps.13314.
ACKNOWLEDGMENTS
Open Access funding enabled and organized by Projekt DEAL.
Funding Information
Open Access funding enabled and organized by Projekt DEAL. WOA Institution: GEORG‐AUGUST‐UNIVERSITAET GOTTINGEN. Blended DEAL: Projekt DEAL
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Pagnin D, de Queiroz V, Pini S, Cassano GB. Efficacy of ECT in depression: a meta‐analytic review. J ECT. 2004;20:13‐20. 10.1097/00124509-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 2. Petrides G, Malur C, Braga RJ, et al. Electroconvulsive therapy augmentation in clozapine‐resistant schizophrenia: a prospective, randomized study. Am J Psychiatry. 2015;172:52‐58. 10.1176/appi.ajp.2014.13060787. [DOI] [PubMed] [Google Scholar]
- 3. Perugi G, Medda P, Toni C, et al. The role of electroconvulsive therapy (ECT) in bipolar disorder: Effectiveness in 522 patients with bipolar depression, mixed‐state, mania and catatonic features. Curr Neuropharmacol. 2017;15:359‐371. 10.2174/1570159X14666161017233642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta‐analysis. Neuropsychopharmacology. 2013;38:2467‐2474. 10.1038/npp.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elias A, Phutane VH, Clarke S, Prudic J. Electroconvulsive therapy in the continuation and maintenance treatment of depression: Systematic review and meta‐analyses. Aust N Z J Psychiatry. 2018;52:415‐424. 10.1177/0004867417743343. [DOI] [PubMed] [Google Scholar]
- 6. Petrides G, Tobias KG, Kellner CH, Rudorfer MV. Continuation and maintenance electroconvulsive therapy for mood disorders: review of the literature. Neuropsychobiology. 2011;64:129‐140. 10.1159/000328943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ward HB, Szabo ST, Rakesh G. Maintenance ECT in schizophrenia: A systematic review. Psychiatry Res. 2018;264:131‐142. 10.1016/j.psychres.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 8. Braga RJ, John M, Schooler NR, et al. Continuation electroconvulsive therapy for patients with clozapine‐resistant schizophrenia: a pilot study. J ECT. 2019;35:156‐160. 10.1097/YCT.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 9. Gill SP, Kellner CH. Clinical practice recommendations for continuation and maintenance electroconvulsive therapy for depression: outcomes from a review of the evidence and a consensus workshop held in Australia in May 2017. J ECT. 2019;35:14‐20. 10.1097/YCT.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 10. Kellner CH, Husain MM, Knapp RG, et al. A novel strategy for continuation ECT in geriatric depression: phase 2 of the PRIDE study. Am J Psychiatry. 2016;173:1110‐1118. 10.1176/appi.ajp.2016.16010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nordenskjöld A, von Knorring L, Ljung T, et al. Continuation electroconvulsive therapy with pharmacotherapy versus pharmacotherapy alone for prevention of relapse of depression: a randomized controlled trial. J ECT. 2013;29:86‐92. 10.1097/YCT.0b013e318276591f. [DOI] [PubMed] [Google Scholar]
- 12. Cabelguen C, Caillet P, Poulet E, et al. Recurrence after stopping maintenance electroconvulsive therapy: a retrospective case series. J ECT. 2020;36:265‐271. 10.1097/YCT.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 13. Martínez‐Amorós E, Serra P, Goldberg X, et al. Evolución clínica tras la discontinuación de la terapia electroconvulsiva de mantenimiento. Estudio de seguimiento retrospectivo. Rev Psiquiatr Salud Ment. 2020;13:5‐10. 10.1016/j.rpsm.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 14. Lapid MI, Seiner S, Heintz H, et al. Electroconvulsive therapy practice changes in older individuals due to COVID‐19: expert consensus statement. Am J Geriatr Psychiatry. 2020;28:1133‐1145. 10.1016/j.jagp.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demas ML. Electroconvulsive therapy and triaging during reduced access and the COVID‐19 pandemic: A personal perspective. J ECT. 2020;36:226‐228. 10.1097/YCT.0000000000000713. [DOI] [PubMed] [Google Scholar]
- 16. Guy W. ECDEU assessment manual for psychopharmacology. US Department of Health, and Welfare. 1976:534‐537.
- 17. Freeman GH, Halton JH. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38:141‐149. [PubMed] [Google Scholar]
- 18. Huuhka K, Viikki M, Tammentie T, et al. One‐year follow‐up after discontinuing maintenance electroconvulsive therapy. J ECT. 2012;28:225‐228. 10.1097/YCT.0b013e3182548f93. [DOI] [PubMed] [Google Scholar]
- 19. Gupta S, Tobiansky R, Bassett P, Warner J. Efficacy of maintenance electroconvulsive therapy in recurrent depression: a naturalistic study. J ECT. 2008;24:191‐194. 10.1097/YCT.0b013e3181608bf2. [DOI] [PubMed] [Google Scholar]
- 20. Lambrichts S, Detraux J, Vansteelandt K, et al. Does lithium prevent relapse following successful electroconvulsive therapy for major depression? A systematic review and meta‐analysis. Acta Psychiatr Scand. 2021;143:294‐306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


