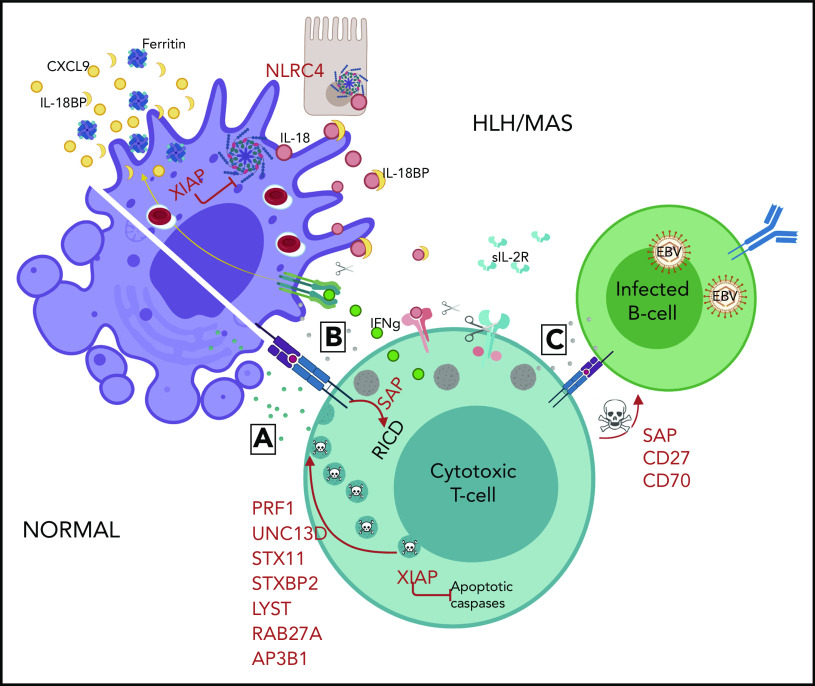
Figure 2.
Mechanisms of genetic HLH predisposition. HLH is thought to develop due to abnormal reciprocal activation of mononuclear phagocytes (MNPs; monocytes, macrophages, and dendritic cells) and type 1 lymphocytes (NK cells and Th1, CD8, and NKT cells). T cells with normal cytotoxic granules release them to induce MNP apoptosis and terminate the synapse, whereas impaired or perforin-deficient granules (gray) cannot terminate MNP activation. Immune synapse prolongation and excess IL-18 (from MNP and/or epithelial sources) both amplify production of lymphocyte cytokines like IFN-γ, which in turn further activates MNPs and promotes hemophagocytosis and release of HLH biomarkers like ferritin, CXCL9, and IL-18BP. Activated lymphocytes upregulate the IL-2 receptor, which is cleaved by proteases released by activated MNPs. The absence of XIAP may permit pathogenic inflammasome activation and lymphocyte apoptosis, while SAP deficiency impairs restimulation-induced cell death (RICD) and, like CD27 and CD70 deficiency, prevents normal killing of EBV-infected B cells.