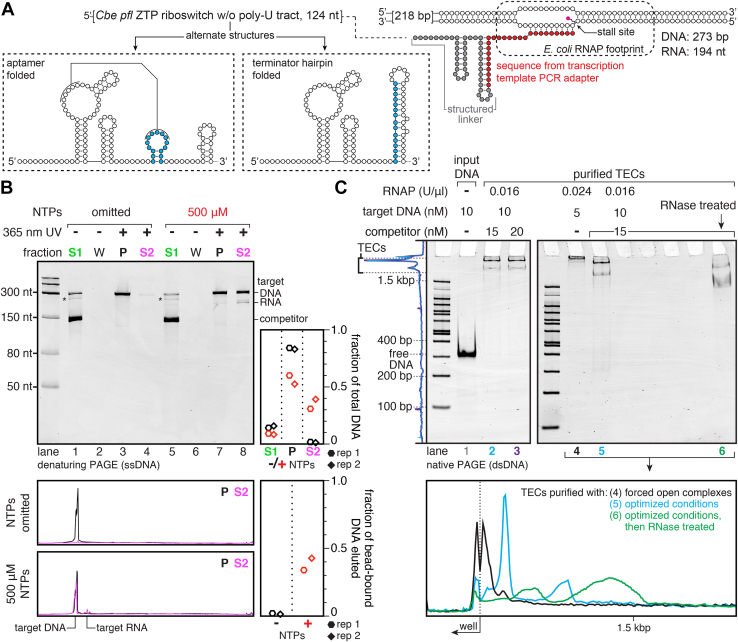
Figure 6.
Purification of TECs containing a 273-bp DNA template and 194-nt RNA.A, overview of the expected TECs. The target RNA contains a variant of the Clostridium beijerinckii pfl ZTP riboswitch with the poly-U tract removed to inactivate the transcription terminator. The pfl riboswitch is tethered to the TEC using a structured linker that sequesters a segment of the transcript derived from a PCR adapter sequence. B, denaturing PAGE analysis of fractions taken during purification of the TECs shown in panel A. Fractions S1, W, P, and S2 were taken as indicated in Figure 3A. The asterisks indicate a minor PCR product that was present in the competitor DNA template preparation. Intensity traces of the target DNA and target RNA bands in fractions P and S2 for each NTP condition are shown below the gel. The plots show the distribution of target DNA across fractions S1, P, and S2, and the fraction of bead-bound DNA that was eluted into fraction S2 after irradiation with 365-nm UV light. C, EMSA analysis of purified TECs. TECs purified using the validated reaction conditions, or with additional competitor DNA, migrate as two major bands (lanes 2, 3, and 5), which may correspond to the alternate riboswitch folds shown in panel A. TECs in lane 4 were prepared using conditions that favor the formation of new open complexes after promoter escape. TECs in lane 6 were prepared using the optimized conditions and treated with a mixture of RNase I, RNaseA, and RNase T1. Empty wells were left between lanes 5 and 6 to avoid RNase cross-contamination. The intensity traces to the left of the gel are for lanes 2 and 3. The intensity traces below the gels are for lanes 4, 5, and 6; for accurate comparison of mobility, these intensity traces were aligned using the sample well (see Experimental procedures). The gels shown in panels B and C are representative of two independent replicates. The replicate of lanes 4, 5, and 6 in panel C is shown in Fig. S4A to illustrate the reproducibility of the complex mobilities. Panels B and C (lanes 1, 2, and 3) are also shown in Fig. S4, C and D with the grayscale adjusted to show trace impurities in the preparation.