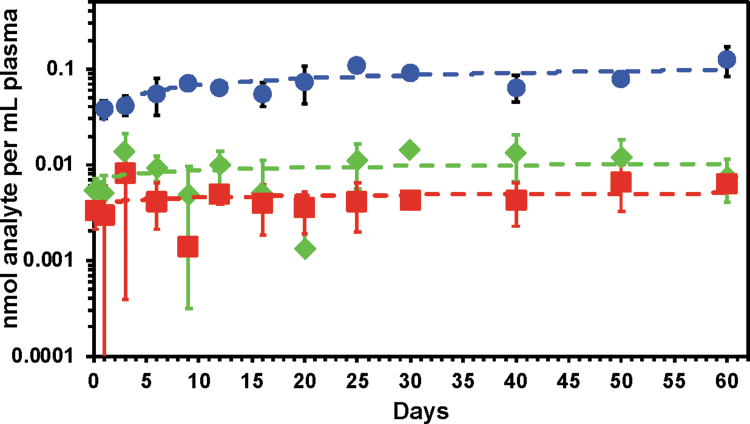
FIG. 1.
The plasma PKs were characterized for the homopolymer drugamer depots with a benzyl carbamate linker, p(Benzyl-TAFMA) with 54.5 drug wt% TAF, or an alkyl carbamate linker, p(Alkyl-TAFMA) with 73 drug wt% TAF. The polymers were formulated in DMSO at the polymer concentration of 625 and 465 mg/mL, respectively. The final dosing volume was 20 μL that corresponded to the 6.77 mg TAF/mouse. Plasma was collected at designated time points (4 h, 1, 3, 6, 9, 12, 16, 20, 25, 30, 40, 50, and 60 days). LC-MS/MS measurements of TFV and TAF were performed with isotope-labeled standards. All animal procedures and handling were performed with the approval of the Institutional Animal Care and Use Committee and kept in accordance with federal and state policies on animal research at the University of Washington. Female BALB/cJ mice, aged 6–8 weeks at time of experiments, were obtained from Jackson Laboratory (Bar Harbor, ME). n = 3 mice per data points. Blue circle: plasma TFV from the p(benzyl-TAFMA), green diamond: plasma TFV from p(alkyl-TAFMA), and red square: plasma TAF from p(alkyl-TAFMA). No TAF was measurable in the p(benzyl-TAFMA) depot. Dash lines are logarithmic trendlines.29 DMSO, dimethyl sulfoxide; LC-MS, liquid chromatography-mass spectrometry; TAF, tenofovir alafenamide; TFV, tenofovir.