Fig. 5. Computed free energy landscape for the conformational transition of CTT and schematic model of dCRY activation.
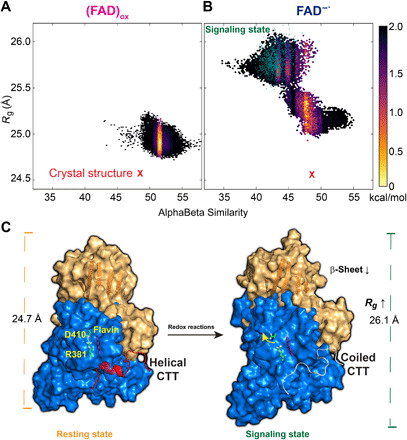
Computed free energy contours from replica-exchange metadynamics simulations are given in the coordinates of the radius gyration (Rg) of the overall protein and the αβ similarity of the CTT for the (FAD)ox (A) and FAD−● (B) states. (C) Schematic model of signaling activation of dCRY. Disengagement of CTT and subsequent helix-to-coil transition are triggered by excited state reduction of the (FAD)ox cofactor to disrupt the Asp410-Arg381 salt bridge to switch on the allosteric communication networks of the N-terminal (brown) and C-terminal (blue) domains through formation of new ion-pair and hydrogen bonds between the two domains. The present simulations reveal that the signaling state shows an increased radius of gyration of dCRY accompanied by reduction in β-sheet content and a disordered CTT conformation in accord with spectroscopic and x-ray scattering experiments.
