Direct human brain recordings reveal how memory rapidly directs vision to support learning.
Abstract
Although the human hippocampus is necessary for long-term memory, controversial findings suggest that it may also support short-term memory in the service of guiding effective behaviors during learning. We tested the counterintuitive theory that the hippocampus contributes to long-term memory through remarkably short-term processing, as reflected in eye movements during scene encoding. While viewing scenes for the first time, short-term retrieval operative within the episode over only hundreds of milliseconds was indicated by a specific eye-movement pattern, which was effective in that it enhanced spatiotemporal memory formation. This viewing pattern was predicted by hippocampal theta oscillations recorded from depth electrodes and by shifts toward top-down influence of hippocampal theta on activity within visual perception and attention networks. The hippocampus thus supports short-term memory processing that coordinates behavior in the service of effective spatiotemporal learning.
INTRODUCTION
The hippocampus is essential for long-term memory (1) and memory-guided behaviors such as spatial navigation (2–4). For example, long-term memory guides visuospatial attention (5, 6) such that past experiences can influence the rapid (∼2 to 5/s) saccadic eye movements needed to sample complex stimuli such as visual scenes (7–9). Yet, the role of the hippocampus in guiding visual sampling might be far more immediate, supporting online representations that emerge across sequential visual fixations and rapidly guide choices of where to look next (10, 11). During the first exposure to complex stimuli, hippocampal lesions disrupt viewing patterns that reflect building a memory for the relations among distinct stimulus features (12, 13), and these viewing patterns correlate with hippocampal activity as measured via functional magnetic resonance imaging (fMRI) in healthy individuals (12, 14). Failure to effectively sample relations among distinct features via eye movements could also underlie impaired perception and short-term retention of complex stimuli identified following hippocampal lesions (15–21). These short-term behavioral deficits are surprising given the standard model of hippocampal involvement in long-term memory and suggest that long-term memory impairments could result from disrupted visual sampling during initial encoding. However, previous studies have been inconclusive in demonstrating that the hippocampus has a short-term role in the rapid formation and online use of memory to guide viewing because those studies lacked the requisite spatial and temporal resolution.
We therefore used intracranial electroencephalography (iEEG) to provide temporally precise measurement of human hippocampal activity aligned to saccadic eye movements during scene memory formation (Fig. 1A). The goal was to test whether hippocampal activity recorded while participants (N = 6) viewed novel scenes reflected the influence of the rapidly forming scene memory on an effective eye-movement pattern that enhanced learning. To achieve this, we identified an eye movement pattern termed “revisitation” while subjects studied novel scenes that reflected the influence of short-term/within-episode memory on viewing. Revisitation signaled effective viewing for memory formation in that it predicted scene-specific spatiotemporal memory as expressed via eye movements during delayed testing. Thus, we hypothesized that revisitation eye movements would temporally dissociate hippocampal iEEG correlates of short-term retrieval, occurring immediately before and therefore initiating the viewing pattern, versus encoding, occurring immediately after the viewing pattern and reflecting its impact on enhanced memory formation.
Fig. 1. Fixation-sequence reinstatement expresses spatiotemporal memory for scenes.

(A) During study, participants viewed scenes while eye-movement tracking recorded their visual fixation sequences. During test, participants viewed repeated or novel scenes with gaze-contingent revealing of scene content such that memory could guide viewing more so than peripheral perceptual information, which was masked. Reinstated fixations during test were coded on the basis of their temporal distance (lag) in the study sequence. Spatiotemporal reinstatement was identified in both forward (+1) and reverse (−1) directions. (B) Participants reinstated fixation sequences in forward and reverse directions, as indicated by the lag conditional viewing probability (lag-CVP) curve. Note that longer fixation-sequence reinstatement results in many +1 or −1 lags given this method of quantification, whereas lags >+1 or <−1 indicate jumping ahead or backward within sequences. Gray lines indicate individual participants. Image credit: K.C. Green, kcgreendotcom.com.
RESULTS
Reinstatement reflects spatiotemporal memory during delayed testing
Our first analysis goal was to establish a measure of scene spatiotemporal memory during delayed testing. Spatiotemporal memory is hippocampal dependent (22, 23) and can be observed for scenes as the tendency for subjects to reinstate during test the scene-specific sequences of visual fixations that they made during study (24). We reasoned that if the hippocampus guides effective visual sampling during initial encoding as indicated by revisitation at study, this behavior should predict spatiotemporal memory as indicated by fixation-sequence reinstatement during delayed testing.
Participants demonstrated robust fixation-sequence reinstatement at test (Fig. 1B). As expected, there was a tendency to reinstate temporally proximal as opposed to distal eye movements (i.e., a contiguity effect) {F3,5 = 5.4, P = 0.01, η2 = 0.52 [95% confidence interval (CI), 0.40 to 0.95]}, with prominent forward (+1 lags) and reverse (−1 lags) reinstatement distinguished from longer lags that were not indicative of spatiotemporal memory. Visual sampling was matched in forward and reverse directions [F1,5 = 2.0, P = 0.22, η2 = 0.28 (95% CI, 0.02 to 0.77)], with comparable contiguity effects in each direction [F3,15 = 0.5, P = 0.71, η2 = 0.08 (95% CI, 0.02 to 0.63)]. Furthermore, although our reliance on an eye-movement measure of spatiotemporal scene memory was motivated by previous findings of heightened sensitivity to hippocampal-dependent memory relative to overt memory judgments (25, 26), reverse fixation-sequence reinstatement was related to overt recognition (see table S1 and fig. S1 for details). Thus, participants exhibited spatiotemporal memory for scenes as reinstatement of fixation sequences in both the forward (+1) and reverse (−1) directions, which is consistent with previous findings in adults without epilepsy (24).
Revisitation during study
During the initial viewing of novel scenes (study phase), we focused on a viewing pattern that has been associated with hippocampal function and the influence of memory retrieval on behavior in multiple previous studies in humans and rodents (27–29). This revisitation viewing pattern occurs when subjects return to fixate a previously viewed location as opposed to moving on to a new location (Fig. 2A). We hypothesized that revisitation would be beneficial for spatiotemporal memory formation and therefore predict fixation-sequence reinstatement during delayed testing.
Fig. 2. Revisitation enhances spatiotemporal memory formation.

(A) Revisitation fixations occurred during study when participants looked back at the previously viewed location as opposed to moving on to a new location. (B) Relative to other fixations at study, participants were more likely to reinstate fixation sequences to revisited locations during test, indicating that revisitation enhanced spatiotemporal memory formation. (C) This relationship between revisitation at study and fixation-sequence reinstatement at test was robust in three independent datasets (D1 to D3) collected from neurologically typical adults. Error bars denote SEM. ∗P < 0.05; ∗∗∗P < 0.001. Dots indicate individual participants. Image credit: K.C. Green, kcgreendotcom.com.
Revisitation occurred for a minority of overall encoding fixations (means = 39% ± 0.02 SEM), indicating that this viewing pattern countered the dominant tendency within scene viewing to sample novel content (30). Despite being less frequent than other (nonrevisitation) fixations, revisitation fixations were made 325 times on average (range, 172 to 590) across all study trials, providing sufficient power to detect relations between this viewing behavior, memory, and brain activity. As hypothesized, revisitation enhanced spatiotemporal memory as measured by fixation-sequence reinstatement during delayed testing (Fig. 2B). Fixations to revisited locations were about twice as likely to be reinstated during retrieval than were other nonrevisited locations in both the forward direction [M = 0.15, SEM = 0.03 versus M = 0.08, SEM = 0.01, respectively, t5 = 4.2, P = 0.008, g = 1.2 (95% CI, 0.3 to 2.5)] and the reverse direction [M = 0.12, SEM = 0.02 versus M = 0.05, SEM = 0.01, respectively, t5 = 4.2, P = 0.009, g = 1.2 (95% CI, 0.3 to 2.3)]. Locations were just as likely to be revisited during encoding of later recognized (M = 40%, SEM = 0.01) as compared to forgotten (M = 38%, SEM = 0.04) scenes [t5 = 0.55, P = 0.61, g = 0.2 (95% CI, −0.7 to 1.1)]. Thus, revisitation fixations reflected effective visual sampling that supports later spatiotemporal reinstatement with or without overt scene recognition.
To confirm the robustness of this finding beyond the sample of participants with epilepsy studied here, we analyzed the relation between revisitation and subsequent fixation-sequence reinstatement in three independent datasets collected from neurologically typical individuals (N = 146 total; see Materials and Methods). Revisitation fixations during encoding significantly predicted later forward and reverse reinstatement (+1 and −1 lags) in each of the three datasets (Fig. 2C; see table S2 for details). This establishes revisitation as a robust viewing pattern during encoding that supports the formation of spatiotemporal memory for scenes.
Controls for stimulus-driven viewing
Revisitation, fixation-sequence reinstatement, and their relationship could be driven by the influence of stimulus features on viewing (31) rather than by the memory processes that we hypothesize. Although visual salience could predict fixation locations made during study at above-chance levels [mean area under the curve (AUC) = 0.88, SEM = 0.01, t5 = 7.7, P = 0.0006, g = 2.0 (95% CI, 0.8 to 3.5)], it could not predict where fixations would be made during gaze-contingent viewing at test [mean AUC = 0.80, SEM = 0.02, t5 = 0.3, P = 0.81, g = 0.04 (95% CI, −0.4 to 0.5); fig. S2A]. Furthermore, revisitation at study was not predicted by the salience of the locations that were fixated [t5 = 0.9, P = 0.42, g = 0.4 (95% CI, −0.5 to 1.2)], whereas it was predicted by the history of previous fixations within a scene [i.e., by short-term memory; t5 = 5.0, P = 0.004, g = 2.1 (95% CI, 0.6 to 3.5); fig. S2B]. Likewise, fixation-sequence reinstatement at test was not predicted by the salience of the locations that were fixated [t5 = 1.1, P = 0.34, g = 0.4 (95% CI, −0.4 to 1.2); fig. S2C]. Reinstatement also did not occur spuriously as a result of short saccade amplitudes [t5 = 1.7, P = 0.15, g = 0.6 (95% CI, −0.3 to 1.6); fig. S2D]. Last, by permuting fixation locations across participants in the healthy control samples, we found that the relation between revisitation at study and fixation-sequence reinstatement at test could not be accounted for by stereotypical viewing patterns (table S2). These control analyses are inconsistent with the interpretation that revisitation and reinstatement were driven by stimulus features or inherent scene memorability and, instead, support our interpretation that these behaviors reflect short-term and long-term memory, respectively.
Hippocampal theta and revisitation
We next addressed the key question of whether activity of the hippocampus predicts the onset of revisitation fixations during study, as would be expected if it contributes to their generation. We focused on theta oscillations recorded from hippocampal iEEG depth electrodes (Fig. 3A), as theta is prominent in the field potential during memory-guided and volitional behavior (3, 32) and coordinates memory processing (33, 34). We found hippocampal theta oscillations during task performance (Fig. 3B), with both low-frequency (centered at 3 Hz) and high-frequency (centered at 8 Hz) theta oscillation peaks (Fig. 3C) often present at the same recording sites (figs. S3 and S4). We measured oscillatory prevalence at participant-defined peaks in the low- and high-theta range, with the majority of subjects having peaks in both ranges (fig. S4).
Fig. 3. Revisitation is predicted by hippocampal theta oscillations.
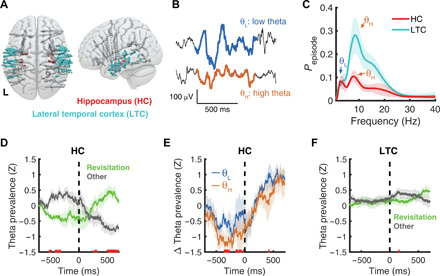
(A) Field potentials were recorded from iEEG electrodes in bilateral hippocampus (HC; red) and in a control region (LTC; cyan). (B) Example low- and high-frequency theta oscillations recording from the hippocampus. (C) Oscillation detection revealed prevalent low- and high-frequency theta oscillations within the hippocampus and LTC. (D) In hippocampus, theta prevalence differed significantly [P < 0.05, false discovery rate (FDR)–corrected] preceding (~500 to 300 ms) and following (~500 to 700 ms) revisitation fixations (time = 0 indicates fixation onset). (E) This relationship was more robust for higher- than lower-frequency theta oscillations. (F) Within LTC, no significant differences in theta prevalence were detected, leading up to revisitation fixations. Shaded regions denote 1 SEM across N = 6 participants.
Modulation of hippocampal theta oscillations before revisitation fixations would support an active role of hippocampus in their generation. In contrast, if the hippocampus were merely to respond to the perceptual input provided by revisitation fixations, then modulation of theta oscillations would be expected only in the postfixation interval. After averaging across low- and high-theta oscillations, we found evidence for hippocampal theta modulation related to revisitation in both the pre- and postfixation intervals (Fig. 3D). From 528 to 300 ms before fixation onset, the prevalence of theta oscillations was significantly less for revisitation than for other fixations [t5 = −3.5, P = 0.02, g = −2.0 (95% CI, −3.9 to −0.5); table S3]. The opposite pattern followed fixation onset, with significantly greater theta prevalence from 495 to 700 ms for revisitation than other fixations [t5 = 4.6, P = 0.006, g = 2.3 (95% CI, 0.9 to 4.2); table S3]. Differences between revisitation and other fixations in the prefixation interval were significantly more pronounced for high than low theta [Fig. 3E; t4 = 4.6, P = 0.01, g = 0.7 (95% CI, 0.2 to 1.5); table S3], whereas high and low theta were similarly affected by revisitation in the postfixation interval. Similar effects were identified in spectral power (fig. S5), indicating that these findings were not specific to our choice of oscillation detection procedure.
Given the rapid dynamics of gaze allocation, modulation of theta oscillations in the prefixation interval could reflect neural processing related to a previous fixation. To account for these potential differences, we repeated our comparison of theta oscillations but considered the type of preceding fixation. We restricted these control analyses to fixations that were exclusively preceded by either revisitation fixations or other (nonrevisitation) fixations. Both control analyses indicated that prefixation differences in theta for revisitation versus other fixations were not driven by modulation of theta by the preceding fixation type (table S4).
We also tested anatomical specificity by using lateral temporal cortex (LTC) as a control site, as theta oscillations in this region behave similarly to hippocampal theta (35), but we did not hypothesize that it would contribute to revisitation. Theta oscillations were robust in LTC, as expected (Fig. 3C). Theta oscillations in the LTC did not predict revisitation fixations (Fig. 3F), demonstrating a degree of anatomical specificity for the evidence of hippocampal theta predicting revisitation fixations.
Prefixation theta decreases indicated both short-term and long-term retrieval
Decreases in hippocampal theta oscillations before revisitation fixations at study provide a signal of short-term/within-episode memory that influences viewing. We next sought to determine whether decreased theta oscillations also reflect retrieval from long-term memory, which we assessed via analysis of prefixation iEEG data during the test phase. The gaze-contingent viewing parameters at test (Fig. 1A) allow long-term memory to guide viewing without interference from peripheral visual inputs. Thus, it is possible to distinguish the general retrieval process of deploying a fixation to a screen location that was viewed during study versus the specific expression of fixation-sequence reinstatement that signals retrieval of spatiotemporal information. We reasoned that this distinction could help identify the nature of the short-term memory retrieval processing that guided revisitation during study (i.e., by determining which of these two types of retrieval at test had similar prefixation activity as revisitation during study).
For fixations to previously studied locations, the prevalence of high-theta oscillations decreased in the prefixation interval (fig. S6). This was specific to high theta, which decreased to a significantly greater degree than low theta [t4 = −14.6, P = 0.0001, g = −5.6 (95% CI, −10.2 to −2.7)]. As shown in fig. S6A, prefixation high theta was similar for revisitation fixations at study and fixations to studied locations at test. High-theta oscillations decreased before fixations at test to studied locations relative to other fixations at study that were not involved in revisitations [fig. S6B; t4 = −4.9, P = 0.008, g = −2.3 (95% CI, −4.8 to −0.6); table S3], as was also the case for revisitation fixations at study (Fig. 3D). In contrast, we found no significant differences in theta before fixation-sequence reinstatement when considering either the forward direction (all |t4| < 0.6, P > 0.58, g < 0.37) or the reverse direction (all |t4| < 0.4, P > 0.74, g < 0.24) relative to nonreinstated fixations made to studied locations. Together, these findings suggest that decreased theta oscillations generally indicated retrieval of fixation-specific but not spatiotemporal information during both short-term retrieval (before revisitation fixations at study) and long-term retrieval (before fixations to studied locations at test).
Cortico-hippocampal interactions supporting revisitation
We hypothesized that if hippocampal activity drives revisitation fixations, then the hippocampus should orchestrate activity within cortical regions that guide eye movements and process the information sampled by fixations. We expected that hippocampal activity before other (nonrevisitation) fixations would indicate bottom-up influences from these regions, reflecting attentional control to sample visual information that feeds forward into hippocampus (31, 36). In contrast, to the extent that revisitation fixations were driven by hippocampal-dependent memory, we expected a shift toward top-down influences from the hippocampus to these regions. We focused our analysis to electrodes within two visually oriented networks (Fig. 4A): the dorsal attention network (DAN), which supports spatial attention and oculomotor control (36), and a visual network (VN) involved in perception. DAN and VN electrodes exhibited low-frequency oscillations, with peak frequencies at 8.8 and 8.2 Hz (near the lower border of the alpha band), respectively (Fig. 4B). These oscillations synchronized with hippocampal theta during encoding, for both the VN [t4 = 3.2, P = 0.03, g = 1.4 (95% CI, 0.1 to 2.7)] and DAN [t4 = 8.4, P = 0.001, g = 3.7 (95% CI, 1.1 to 6.4)] (Fig. 4, C and D). These findings parallel demonstrations of visual theta in rodents (37) and nonhuman primates (38) and suggest that the hippocampus is linked to rhythmic perception and attention (39, 40).
Fig. 4. Directed influence of hippocampus on cortical network theta oscillations before fixations.

(A) Electrode locations in the DAN and VN regions of interest. (B) Both networks exhibited peak oscillations (Pepisode) in the high-theta range. (C) Time-frequency plots indicate theta synchrony of hippocampus with the DAN and VN during encoding (time = 0 indicates fixation onset). A transparency mask highlights significant time-frequency points (P < 0.05, FDR-corrected). (D) Synchrony was consistently high across the pre- and postfixation interval. (E) Directional theta interactions between the hippocampus and cortical networks indicated that in-formation flow (PSI) was directed from the VN to the hippocampus preceding nonrevisitation fixations (left). Information flowed from the hippocampus to the DAN preceding both revisitation and other fixations (right). (F) Information flow from the VN to the hippocampus was significantly greater preceding revisitation fixations than other fixations. All plots depict data from N = 5 participants with electrode contacts in these cortical networks and the hippocampus. Shaded regions depict 1 SEM. ***P < 0.05, FDR-corrected.
We next tested the hypothesis that the hippocampus drives activity of these systems and that it does so to a greater extent during revisitation than other fixations. We calculated the phase slope index (PSI), a measure of directed information flow, between hippocampus and both systems. For the VN, there was significant bottom-up information flow to the hippocampus before participants made other (nonrevisitation) fixations (Fig. 4E, significantly negative PSI) [t4 = −4.6, P = 0.01, g = −2.1 (95% CI, −3.7 to −0.4)] that shifted to more top-down information flow from hippocampus to visual system before revisitation fixations [Fig. 4F; t4 = 4.7, P = 0.009, g = 2.0 (95% CI, 0.6 to 3.9); table S3]. We observed significant information flow from hippocampus to DAN before both revisitation fixations [t4 = 2.9, P = 0.04, g = 1.3 (95% CI, 0.03 to 2.5)] and other fixations [t4 = 4.5, P = 0.01, g = 2.0 (95% CI, 0.4 to 3.6)] without modulation by fixation type (Fig. 4E). Thus, hippocampal coordination of DAN occurred before all fixations, whereas reversal of the typical bottom-up flow of information from the visual system to the hippocampus uniquely occurred before revisitation fixations.
DISCUSSION
Previous findings of hippocampal involvement in guiding eye movements by long-term memory (7–9, 41) support the standard model of hippocampal long-term memory function (1). In contrast, the current focus on initial learning permitted testing of a far more immediate hippocampal contribution to short-term (i.e., within-episode) memory. We identified hippocampal contributions to memory processing across rapid gaze fixations, highlighting an active, immediate role of the hippocampus to guide forthcoming fixations in a manner conducive to learning. Revisitation fixations provided temporally precise behavioral markers of this process. Revisitation fixations countered the typical pattern of looking toward novel rather than previously viewed scene content (30), suggesting sporadic guidance by memory retrieval and enhanced learning, as indicated by better subsequent spatiotemporal memory. This interpretation is consonant with shifts in hippocampal states from pronounced theta oscillations during novel exploration to theta-free epochs marked with sharp-wave ripple events (42) thought to support replay of previous experience (43). Eye-movement tracking therefore provided a marker of memory processing, with the requisite temporal precision to resolve the behavioral ramifications of dynamic changes in hippocampal activity reflecting encoding versus retrieval that occur rapidly over a brief interval (41, 44), even during a single episode during which learning occurs for a novel stimulus.
We identified reductions in theta before initiating revisitation followed by increases in theta during the fixation period, when visual information is processed. Demands for associative or contextual processing can determine when increases rather than decreases in theta accompany successful encoding (33). This issue is further complicated as previous work typically has not separated theta oscillations from changes or tilts in the overall power spectrum caused by altered excitability during memory (45). As we specifically measured theta oscillations separately from broadband changes in the power spectrum, the observed modulations in theta during revisitations thus reflect synchronous theta activity rather than differences in overall excitability. One interpretation of our findings is that increases in theta following revisitation reflect a mechanism by which the content encoded across multiple fixations becomes associated into a sequential representation, potentially through coordination of gamma band activity (46, 47). This mechanism is distinct from theta phase resets initiated by saccades to novel content (41, 48), which are believed to promote memory formation by enhancing plasticity when visual inputs become associated in the hippocampus. Theta bouts in the hippocampus may thus serve as a mechanism to define individual fixation sequences or “viewing episodes” that are later reinstated to support accurate recognition.
These findings also situate the hippocampus within a distributed system for visual cognition, attention, and memory. The hippocampus is thought to provide top-down influence on visual perceptual networks in some circumstances (11, 49), particularly when long-term memory can guide perceptual expectations (50). Similarly, the cortical oculomotor system that guides visuospatial attention (36) is thought to be driven mainly by perceptual and semantic scene features (31, 51), although hippocampal-dependent long-term memory can guide attention (5–9, 26, 41, 52). Our findings provide new insights into hippocampal influence on perceptual and attention systems by characterizing the time course of relevant interregional interactions with high temporal precision. By identifying enhanced hippocampal influence on perceptual and attention networks in relation to short-term/within-episode memory, our findings expand the scope of hippocampal influence on these networks beyond long-term memory. Furthermore, we found that the nature of hippocampal influence varied rapidly with eye-movement behavior. That is, the expected bottom-up flow of information from the VN to hippocampus was abated immediately before revisitation fixations, and the directed influence of the hippocampus on the attention network proceeded rather than followed individual fixations. Thus, directional interactions of the hippocampus with perceptual and attention networks vary rapidly to effectively coordinate ongoing behavior even during new learning when hippocampal involvement can reflect only short-term, rather than long-term, memory.
Selecting where to direct gaze is akin to a decision-making process, which can be informed by memory-guided predictions of future states (53). Although our experiment was not able to test relevant prefrontal cortex contributions due to insufficient coverage by recording electrodes, prefrontal representations of task goals interact with hippocampal memory processes during memory-guided decisions (54). These prefrontal-hippocampal interactions are thought to mediate memory-guided decision-making behaviors such as revisitation across species (27, 28).
Because hippocampal influences on visual behavior occurred during initial scene viewing in our experiment, they cannot be explained by long-term memory but rather must reflect the active guidance of attention and perception by online hippocampal representations. The similarity of high-theta dynamics when the gaze was guided by either short-term or long-term retrieval indicates a common process of the hippocampus in memory-guided viewing, irrespective of delay. The long and the short of hippocampal function may therefore be its critical role in the effective coordination of attention and perception during learning, explaining why hippocampal damage and dysfunction impairs long-term memory (1) as well as perception and short-term retention (15–18).
MATERIALS AND METHODS
Participants
We enrolled six participants (three females) with medically refractory epilepsy from the Northwestern Memorial Hospital Comprehensive Epilepsy Center (Chicago, IL). All participants had depth electrodes implanted as part of neurosurgical monitoring before elective surgery. Inclusion criteria for this study were implantation of electrodes into the hippocampus. The average age of participants was 29 (range, 24 to 38) years. Neuropsychological testing during presurgical workup found the average FSIQ (full-scale intelligent quotient) [WAIS-IV (Wechsler Adult Intelligence Scale-IV)] to be 98.3 (range, 76 to 112). Inspection of neuroimaging revealed no evidence of hippocampal sclerosis in any participants. Etiology was typically found to be cortical dysplasia (N = 4), with seizure onset zones identified within mesial (N = 2), lateral (N = 1), or multiple (N = 1) temporal lobe structures. Additional seizure onset zones were localized to the cingulate cortex (N = 1) and to nodular heterotopic gray matter lining the temporal and occipital horns of the lateral ventricle (N = 1). Written informed consent was acquired before participation in the research protocol in accordance with the Northwestern University Institutional Review Board.
iEEG recordings
Stereotactic EEG electrodes (contacts spaced 5 to 10 mm apart; AD-TECH Medical Instrument Co., Racine, WI) targeted brain structures based on clinical needs but provided coverage in the hippocampus as well as the dorsal attention and VNs beyond the seizure onset zone. Electrophysiological data were recorded with a clinical reference and ground consisting of either a surgically implanted electrode strip facing the scalp or a scalp electrode. Recordings were made using a Nihon Kohden amplifier with a sampling rate of 1 to 2 kHz per clinical needs. Recorded signals were bandpass-filtered from 0.6 to 600 Hz and re-referenced offline to a bipolar montage computed using adjacent electrode contacts. Line noise and harmonics (60, 120, and 180 Hz) were removed with a discrete Fourier transform filter. To rule out the possibility that epileptiform activity influenced our oscillation detection analyses, all data within 1 s of interictal epileptiform discharges were excluded from analysis using an automated algorithm that detects large amplitude increases in high-frequency activity (55).
Electrode localization
Postimplant computed tomography (CT) was coregistered to presurgical T1 weighted structural MRIs using SPM12 (56). All T1-weighted MRI scans were normalized to MNI (Montreal Neurological Institute) space by using a combination of affine and nonlinear registration steps, bias correction, and segmentation into gray matter, white matter, and cerebrospinal fluid components. Deformations from the normalization procedure were applied to individual electrode locations identified on postimplant CT images or structural images using Bioimage Suite (https://medicine.yale.edu/bioimaging/suite/). Bipolar pairs with at least one contact within either hippocampus, DAN, or VN (Fig. 4) were analyzed. Electrode contacts in hippocampus were verified by visual inspection of coregistered anatomical scans and additionally included contacts directly adjacent to hippocampal gray matter in the hippocampal-amygdala transition area in two participants. Contacts in DAN and VN were defined on the basis of cortical parcellations of Yeo and colleagues (57). Maximum probability estimates from the Harvard-Oxford atlas (58) defined contacts within the LTC (see fig. S7 for additional information).
Eye tracking
We recorded eye movements at 500 Hz using an EyeLink 1000 remote tracking system (SR Research, Ontario, Canada). Continuous eye-movement records were parsed into fixation, saccade, and blink events. Motion (0.15°), velocity (30°/s), and acceleration (8000°/s2) thresholds indicated saccade events. Blinks were determined by pupil size, and the remaining epochs below detection thresholds were classified as fixations. The location of each fixation event was computed as the average gaze position throughout the duration of the fixation. The eye-tracking camera was mounted beneath the computer monitor that displayed the task, which was affixed to a movable arm to allow positioning directly (~60 cm) in front of the patient’s eyes.
Recognition memory task
A scene recognition task was designed using Presentation software (version 18.0, Neurobehavioral Systems Inc., Berkeley, CA). The task consisted of eight blocks, in which the participant studied a sequence of 24 images followed by a recognition test. The images contained common objects in naturally occurring contexts (59) restricted to scenes containing people, animals, or food (eight scenes of each category per block). From a total pool of 384 images, scenes were randomly assigned as targets (presented at study and test) or lures (presented only at test). During study, scenes were displayed for 3 s, followed by a randomly jittered intertrial interval of 0.8 to 1.2 s (uniformly distributed). We presented a centrally located fixation cross for 0.5 s before each scene appeared to alert the participant to the upcoming trial. Scene images were displayed on the computer monitor and subtended approximately 24° by 24° of visual angle.
During test, participants viewed 24 novel and 24 repeated scenes from the previous encoding block in pseudorandom order. Novel scenes were matched for content (they contained the same type of objects) to the repeated scenes but were not previously viewed in the task. The test phase of the task used a gaze-contingent design to allow memory to guide visual sampling. Scene content was masked by a gray overlay, which was made transparent through a Gaussian kernel (μ = 0, σ = 1.25° of visual angle) centered at the current gaze position. An exponential moving average (α = 0.6) reduced transient shifts in the position of the revealed window. Before each test trial, a fixation cross appeared for 0.5 s at a cued location that indicated where to begin visual search. For a given scene, the cue appeared at the center of an object with either the highest or lowest visual salience, as defined by the DeepGaze II model (60). Gaze-contingent search followed until the participant indicated by button press that the scene was either repeated or novel. Test trials were separated by a 0.8- to 1.2-s intertrial interval. Because behavioral performance (d′) did not differ between high and low salience cues [t5 = −1.0, P = 0.36, g = −0.1 (95% CI, −0.4 to 0.2)], we report analyses that collapse across these two conditions.
Throughout the task, eye-tracking validation was performed before study and test phases of every block. If the average error exceeded 1.5° of visual angle on the five-point calibration test, the eye tracker was recalibrated before continuing with the task.
Eye-movement analysis
To measure reinstatement of fixation sequences during scene recognition, we computed lag conditional viewing probability (lag-CVP) curves, a recently developed measure of gaze reinstatement (24). Briefly, gaze fixations made during encoding were serialized. Reinstatement during retrieval was measured by computing the probability of viewing a region of the scene, conditional on the distance (lag) of the previous fixation in the encoding sequence. Fixations at retrieval were matched to the encoding fixations using a nearest neighbor approach (using Euclidean distance), with a threshold of 2° of visual angle. Using this approach, it is possible for very proximal fixations at test to match distinct encoding fixations. However, less than 2% of fixations that were well within the gaze-contingent viewing window (i.e., less than 1.25° of visual angle apart) were assigned to distinct encoding fixations. Lags were computed on the basis of this updated sequence. Reinstatement was defined as lag 1 transitions in forward (+1) and reverse (−1) directions.
Revisitation fixations were defined as gaze fixations made during scene encoding when the gaze returned to a previously fixated location. As in the definition of reinstatement, a threshold of 2° of visual angle was used to assign fixations to specific locations. To examine the influence of revisitation on reinstatement, fixated locations at study were labeled as involved in subsequent forward (+1 lags) or reverse (−1 lags) reinstatement, or not. Then, the probability of reinstatement was compared for revisited and nonrevisited locations. To generalize our behavioral findings outside the sample of epileptic patients, we replicated these analyses in three independent datasets that used concurrent eye tracking with repeated viewing of scenes (61–63). We further assessed the influence of stimulus features on viewing behaviors (i.e., the relation between revisitation and reinstatement) using a permutation procedure where, rather than using fixation sequences from the same participant, sequences were shuffled across participants during study. The average amount of reinstatement for revisited and other fixations at study was used as an estimate of stimulus-driven viewing effects.
We assessed the ability of visual features to predict gaze behavior by using the DeepGaze II model (60) to predict which locations within a scene would be fixated. Performance of this model was compared to a center bias model, computed as the average viewing probability over all other scenes within a given phase of the task. To compute this null model, we convolved binary images indicating fixation locations with a Gaussian kernel with SD of 1° of visual angle. We computed the AUC metric (64) to evaluate model performance on distinct phases of the task.
Analysis of theta oscillations
As observed in humans, nonhuman primates, and bats (48, 65, 66), theta oscillations were transient in nature (Fig. 3B). We therefore quantified theta oscillations using Pepisode, which indicates the probability of an oscillation being present, using an automated oscillation detection algorithm (67, 68). Following this approach, we defined oscillatory episodes as temporal epochs that exhibited high spectral power within a specified frequency band that was sustained for at least three full cycles. Estimates of spectral power were obtained through Morlet wavelet convolution (six cycles) at 50 frequencies from 1 to 40 Hz. We adapted a robust regression procedure (69) to fit the log of power and frequency across the entire recording session. The mean power for a given frequency was used to build a χ2(2) distribution to represent the contribution of 1/f signal to power at a given frequency. Epochs with spectral power above 95% of this distribution and duration of at least three cycles were considered oscillations. As theta oscillations were prevalent at lower (~3 Hz) and higher (~8 Hz) frequencies (see fig. S4), we selected the peak theta frequency in each band (using 5 Hz to separate low and high theta), if present at a given electrode contact. Having identified bouts of theta oscillations, we measured the proportion of time that oscillations were present (Pepisode) for different event types of interest (67). Pepisode was then used as a dependent measure for statistical analysis. To determine whether our findings were specific to our oscillation detection method, we also compared raw changes in spectral power from 750 ms before to 750 ms after fixation onset. A multitaper approach with a Hanning taper was used to estimate the time-frequency representation from 1 to 200 Hz, using a moving window of two cycles in duration, with spectral smoothing of 2 Hz. Differences in spectral power across condition were then compared using nonparametric statistical testing, as described below.
Directed coupling between the hippocampus and visual systems
We measured synchrony between local field potentials recorded from the hippocampus and visual regions using the phase-locking value (70). For each pairwise combination of contacts in the hippocampus and cortical regions, we computed synchrony as described above. We standardize these measures across electrode contacts for group comparison using a permutation approach (N = 1000). We constructed a null distribution by shuffling the hippocampal phase estimates across trials and z-transformed observed values based on the mean and SD of this null distribution. Before group analysis, all connections between the hippocampus and a given cortical region of interest were averaged. Statistical analysis was then performed using nonparametric tests as described below.
After establishing synchrony between the hippocampus and cortical sites, we tested for potential directional interactions using the PSI (71). We computed the PSI in a time-resolved manner in the moments surrounding both revisitation and other fixations. For two recorded signals, PSI is based on the slope of phase differences (between two recorded sources) with increasing frequency. To standardize PSI values, phase values were permuted (N = 1000) and a distribution was generated to estimate the mean and SD under the null hypothesis of no relation between frequency and phase differences. These standardized scores were then submitted to nonparametric permutation statistics, as described below.
Quantification and statistical analysis
Data are reported as mean ± SE. Nonparametric (Wilcoxon signed rank, Wilcoxon rank sum, and Kruskal-Wallis) statistical tests were used for tests on bounded observations (e.g., prevalence of oscillations). Parametric tests (paired or one-sample t tests) were applied to data with normally distributed residuals. Nonparametric permutations tests at the subject level (72) with false discovery rate (FDR) correction for multiple comparisons (73) were applied for all time-series and time-frequency analyses, unless stated otherwise. Alpha was set to 0.05 for determining statistical significance. We performed additional nonparametric statistical tests where we permutated data at the fixation level, across conditions. These analyses qualitatively replicated the primary findings (see table S3). Nonetheless, further replication in a larger cohort would be beneficial given the sample size. Repeated-measures analyses of variance (ANOVAs) were used for behavior analysis. Hedges’ g (74) was used to estimate effect sizes, with values estimated at peaks for time-series analysis. For these tests involving multiple comparisons, estimates are reported for significant effects and are likely biased as a result.
Acknowledgments
We thank the patients of the Northwestern University Comprehensive Epilepsy Center and their families for their efforts to facilitate this research. We thank M. Aly for comments on a previous version of the manuscript. Funding: This work was supported by National Institute of Neurological Disorders and Stroke grants T32NS047987 and R01NS113804. Author contributions: Conceptualization: J.E.K. and J.L.V.; formal analysis: J.E.K.; data collection: J.E.K. and J.L.V.; writing—original draft preparation: J.E.K.; writing—review and editing: J.E.K., S.V., S.S., J.M.R., and J.L.V.; resources: S.V., S.S., J.M.R., and J.L.V.; supervision: J.L.V. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Raw data and code necessary to reproduce this work are freely available (https://doi.org/10.5281/zenodo.4728229).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/25/eabf7144/DC1
REFERENCES AND NOTES
- 1.Squire L. R., Zola-Morgan S., The medial temporal lobe memory system. Science 253, 1380–1386 (1991). [DOI] [PubMed] [Google Scholar]
- 2.Miller J. F., Neufang M., Solway A., Brandt A., Trippel M., Mader I., Hefft S., Merkow M., Polyn S. M., Jacobs J., Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science 342, 1111–1114 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellmund J. L. S., Gardenfors P., Moser E. I., Doeller C. F., Navigating cognition: Spatial codes for human thinking. Science 362, eaat6766 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Lopes-Dos-Santos V., van de Ven G. M., Morley A., Trouche S., Campo-Urriza N., Dupret D., Parsing hippocampal theta oscillations by nested spectral components during spatial exploration and memory-guided behavior. Neuron 100, 940–952.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun M. M., Turk-Browne N. B., Interactions between attention and memory. Curr. Opin. Neurobiol. 17, 177–184 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Summerfield J. J., Lepsien J., Gitelman D. R., Mesulam M. M., Nobre A. C., Orienting attention based on long-term memory experience. Neuron 49, 905–916 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Leonard T. K., Hoffman K. L., Sharp-wave ripples in primates are enhanced near remembered visual objects. Curr. Biol. 27, 257–262 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Hannula D. E., Ranganath C., The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron 63, 592–599 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wynn J. S., Ryan J. D., Buchsbaum B. R., Eye movements support behavioral pattern completion. Proc. Natl. Acad. Sci. U.S.A. 117, 6246–6254 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voss J. L., Bridge D. J., Cohen N. J., Walker J. A., A closer look at the hippocampus and memory. Trends Cogn. Sci. 21, 577–588 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nobre A. C., Stokes M. G., Premembering experience: A hierarchy of time-scales for proactive attention. Neuron 104, 132–146 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voss J. L., Warren D. E., Gonsalves B. D., Federmeier K. D., Tranel D., Cohen N. J., Spontaneous revisitation during visual exploration as a link among strategic behavior, learning, and the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 108, E402–E409 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas H. D., Duff M. C., Cohen N. J., The hippocampus promotes effective saccadic information gathering in humans. J. Cogn. Neurosci. 1–16 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Voss J. L., Cohen N. J., Hippocampal-cortical contributions to strategic exploration during perceptual discrimination. Hippocampus 27, 642–652 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeidman P., Maguire E. A., Anterior hippocampus: The anatomy of perception, imagination and episodic memory. Nat. Rev. Neurosci. 17, 173–182 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aly M., Ranganath C., Yonelinas A. P., Detecting changes in scenes: The hippocampus is critical for strength-based perception. Neuron 78, 1127–1137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham K. S., Scahill V. L., Hornberger M., Barense M. D., Lee A. C., Bussey T. J., Saksida L. M., Abnormal categorization and perceptual learning in patients with hippocampal damage. J. Neurosci. 26, 7547–7554 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekstrom A. D., Yonelinas A. P., Precision, binding, and the hippocampus: Precisely what are we talking about? Neuropsychologia 138, 107341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A. C., Bussey T. J., Murray E. A., Saksida L. M., Epstein R. A., Kapur N., Hodges J. R., Graham K. S., Perceptual deficits in amnesia: Challenging the medial temporal lobe ‘mnemonic’ view. Neuropsychologia 43, 1–11 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Olson I. R., Moore K. S., Stark M., Chatterjee A., Visual working memory is impaired when the medial temporal lobe is damaged. J. Cogn. Neurosci. 18, 1087–1097 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Olson I. R., Page K., Moore K. S., Chatterjee A., Verfaellie M., Working memory for conjunctions relies on the medial temporal lobe. J. Neurosci. 26, 4596–4601 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palombo D. J., Di Lascio J. M., Howard M. W., Verfaellie M., Medial temporal lobe amnesia is associated with a deficit in recovering temporal context. J. Cogn. Neurosci. 31, 236–248 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Goyal A., Miller J., Watrous A. J., Lee S. A., Coffey T., Sperling M. R., Sharan A., Worrell G., Berry B., Lega B., Jobst B. C., Davis K. A., Inman C., Sheth S. A., Wanda P. A., Ezzyat Y., Das S. R., Stein J., Gorniak R., Jacobs J., Electrical stimulation in hippocampus and entorhinal cortex impairs spatial and temporal memory. J. Neurosci. 38, 4471–4481 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kragel J. E., Voss J. L., Temporal context guides visual exploration during scene recognition. J. Exp. Psychol. Gen. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannula D. E., Althoff R. R., Warren D. E., Riggs L., Cohen N. J., Ryan J. D., Worth a glance: Using eye movements to investigate the cognitive neuroscience of memory. Front. Hum. Neurosci. 4, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen R. K., Moses S. N., Riggs L., Ryan J. D., The hippocampus supports multiple cognitive processes through relational binding and comparison. Front. Hum. Neurosci. 6, 146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redish A. D., Vicarious trial and error. Nat. Rev. Neurosci. 17, 147–159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J. X., Cohen N. J., Voss J. L., Covert rapid action-memory simulation (CRAMS): A hypothesis of hippocampal-prefrontal interactions for adaptive behavior. Neurobiol. Learn. Mem. 117, 22–33 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckner R. L., The role of the hippocampus in prediction and imagination. Annu. Rev. Psychol. 61, 27–48 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Klein R. M., Inhibition of return. Trends Cogn. Sci. 4, 138–147 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Itti L., Koch C., Computational modelling of visual attention. Nat. Rev. Neurosci. 2, 194–203 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Pacheco Estefan D., Zucca R., Arsiwalla X., Principe A., Zhang H., Rocamora R., Axmacher N., Verschure P. F. M. J., Volitional learning promotes theta phase coding in the human hippocampus. Proc. Natl. Acad. Sci. U.S.A. 118, e2021238118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herweg N. A., Solomon E. A., Kahana M. J., Theta oscillations in human memory. Trends Cogn. Sci. 24, 208–227 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buzsaki G., Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Bush D., Bisby J. A., Bird C. M., Gollwitzer S., Rodionov R., Diehl B., McEvoy A. W., Walker M. C., Burgess N., Human hippocampal theta power indicates movement onset and distance travelled. Proc. Natl. Acad. Sci. U.S.A. 114, 12297–12302 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbetta M., Akbudak E., Conturo T. E., Snyder A. Z., Ollinger J. M., Drury H. A., Linenweber M. R., Petersen S. E., Raichle M. E., Van Essen D. C., Shulman G. L., A common network of functional areas for attention and eye movements. Neuron 21, 761–773 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Fournier J., Saleem A. B., Diamanti E. M., Wells M. J., Harris K. D., Carandini M., Mouse visual cortex is modulated by distance traveled and by theta oscillations. Curr. Biol. 30, 3811–3817.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spyropoulos G., Bosman C. A., Fries P., A theta rhythm in macaque visual cortex and its attentional modulation. Proc. Natl. Acad. Sci. U.S.A. 115, E5614–E5623 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiebelkorn I. C., Pinsk M. A., Kastner S., A dynamic interplay within the frontoparietal network underlies rhythmic spatial attention. Neuron 99, 842–853.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helfrich R. F., Fiebelkorn I. C., Szczepanski S. M., Lin J. J., Parvizi J., Knight R. T., Kastner S., Neural mechanisms of sustained attention are rhythmic. Neuron 99, 854–865.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kragel J. E., VanHaerents S., Templer J. W., Schuele S., Rosenow J. M., Nilakantan A. S., Bridge D. J., Hippocampal theta coordinates memory processing during visual exploration. eLife 9, e52108 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.J. O’Keefe, L. Nadel, The Hippocampus as a Cognitive Map (Oxford Univ. Press, 1978). [Google Scholar]
- 43.Vaz A. P., Wittig J. H. Jr., Inati S. K., Zaghloul K. A., Replay of cortical spiking sequences during human memory retrieval. Science 367, 1131–1134 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez-Madrona V. J., Perez-Montoyo E., Alvarez-Salvado E., Moratal D., Herreras O., Pereda E., Mirasso C. R., Canals S., Different theta frameworks coexist in the rat hippocampus and are coordinated during memory-guided and novelty tasks. eLife 9, e57313 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao R., Peterson E. J., Voytek B., Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage 158, 70–78 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Lisman J. E., Jensen O., The θ-γ neural code. Neuron 77, 1002–1016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heusser A. C., Poeppel D., Ezzyat Y., Davachi L., Episodic sequence memory is supported by a theta-gamma phase code. Nat. Neurosci. 19, 1374–1380 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jutras M. J., Fries P., Buffalo E. A., Oscillatory activity in the monkey hippocampus during visual exploration and memory formation. Proc. Natl. Acad. Sci. U.S.A. 110, 13144–13149 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turk-Browne N. B., The hippocampus as a visual area organized by space and time: A spatiotemporal similarity hypothesis. Vision Res. 165, 123–130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hindy N. C., Avery E. W., Turk-Browne N. B., Hippocampal-neocortical interactions sharpen over time for predictive actions. Nat. Commun. 10, 3989 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henderson J. M., Hayes T. R., Meaning-based guidance of attention in scenes as revealed by meaning maps. Nat. Hum. Behav. 1, 743–747 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wynn J. S., Shen K., Ryan J. D., Eye movements actively reinstate spatiotemporal mnemonic content. Vision 3, 21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stachenfeld K. L., Botvinick M. M., Gershman S. J., The hippocampus as a predictive map. Nat. Neurosci. 20, 1643–1653 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Minxha J., Mosher C., Morrow J. K., Mamelak A. N., Adolphs R., Gothard K. M., Rutishauser U., Fixations gate species-specific responses to free viewing of faces in the human and macaque amygdala. Cell Rep. 18, 878–891 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janca R., Jezdik P., Cmejla R., Tomasek M., Worrell G. A., Stead M., Wagenaar J., Jefferys J. G. R., Krsek P., Komarek V., Jiruska P., Marusic P., Detection of interictal epileptiform discharges using signal envelope distribution modelling: Application to epileptic and non-epileptic intracranial recordings. Brain Topogr. 28, 172–183 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Ashburner J., Friston K., Multimodal image coregistration and partitioning—A unified framework. Neuroimage 6, 209–217 (1997). [DOI] [PubMed] [Google Scholar]
- 57.Yeo B., Krienen F., Sepulcre J., Sabuncu M., Lashkari D., Hollinshead M., Roffman J., Smoller J., Zöllei L., Polimeni J., The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desikan R. S., Segonne F., Fischl B., Quinn B. T., Dickerson B. C., Blacker D., Buckner R. L., Dale A. M., Maguire R. P., Hyman B. T., Albert M. S., Killiany R. J., An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006). [DOI] [PubMed] [Google Scholar]
- 59.T. Y. Lin, M. Maire, S. Belongie, J. Hays, P. Perona, D. Ramanan, P. Dollar, C. L. Zitnick, Microsoft COCO: Common objects in context, in Computer Vision – ECCV 2014. ECCV 2014. Lecture Notes in Computer Science, D. Fleet, T. Pajdla, B. Schiele, T. Tuytelaars, Eds. (Springer, 2014), vol. 8693, pp. 740–755. [Google Scholar]
- 60.M. Kümmerer, T. S. A. Wallis, M. Bethge, DeepGaze II: Reading fixations from deep features trained on object recognition. arXiv:1610.01563 [cs.CV] (5 October 2016).
- 61.Bylinskii Z., Isola P., Bainbridge C., Torralba A., Oliva A., Intrinsic and extrinsic effects on image memorability. Vision Res. 116, 165–178 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Kaspar K., Konig P., Overt attention and context factors: The impact of repeated presentations, image type, and individual motivation. PLOS ONE 6, e21719 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaspar K., Konig P., Viewing behavior and the impact of low-level image properties across repeated presentations of complex scenes. J. Vis. 11, 26 (2011). [DOI] [PubMed] [Google Scholar]
- 64.Kummerer M., Wallis T. S. A., Bethge M., Information-theoretic model comparison unifies saliency metrics. Proc. Natl. Acad. Sci. U.S.A. 112, 16054–16059 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vass L. K., Copara M. S., Seyal M., Shahlaie K., Farias S. T., Shen P. Y., Ekstrom A. D., Oscillations go the distance: Low-frequency human hippocampal oscillations code spatial distance in the absence of sensory cues during teleportation. Neuron 89, 1180–1186 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ulanovsky N., Moss C. F., Hippocampal cellular and network activity in freely moving echolocating bats. Nat. Neurosci. 10, 224–233 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Whitten T. A., Hughes A. M., Dickson C. T., Caplan J. B., A better oscillation detection method robustly extracts EEG rhythms across brain state changes: The human alpha rhythm as a test case. Neuroimage 54, 860–874 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Caplan J. B., Madsen J. R., Raghavachari S., Kahana M. J., Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J. Neurophysiol. 86, 368–380 (2001). [DOI] [PubMed] [Google Scholar]
- 69.Donoghue T., Haller M., Peterson E. J., Varma P., Sebastian P., Gao R., Noto T., Lara A. H., Wallis J. D., Knight R. T., Shestyuk A., Voytek B., Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 23, 1655–1665 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lachaux J. P., Rodriguez E., Martinerie J., Varela F. J., Measuring phase synchrony in brain signals. Hum. Brain Mapp. 8, 194–208 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nolte G., Ziehe A., Nikulin V. V., Schlogl A., Kramer N., Brismar T., Muller K. R., Robustly estimating the flow direction of information in complex physical systems. Phys. Rev. Lett. 100, 234101 (2008). [DOI] [PubMed] [Google Scholar]
- 72.Maris E., Oostenveld R., Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Benjamini Y., Yekutieli D., The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 29, 1165–1188 (2001). [Google Scholar]
- 74.Hedges L. V., Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 6, 107–128 (1981). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/25/eabf7144/DC1


