Abstract
Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection has caused a global pandemic in the past year, which poses continuing threat to human beings. To date, more than 3561 mutations in the viral spike protein were identified, including 2434 mutations that cause amino acid changes with 343 amino acids located in the viral receptor-binding domain (RBD). Among these mutations, the most representative ones are substitution mutations such as D614G, N501Y, Y453F, N439K/R, P681H, K417N/T, and E484K, and deletion mutations of ΔH69/V70 and Δ242–244, which confer the virus with enhanced infectivity, transmissibility, and resistance to neutralization. In this review, we discussed the recent findings of SARS-CoV-2 for highlighting mutations and variants on virus transmissibility and pathogenicity. Moreover, several suggestions for prevention and controlling the pandemic are also proposed
Keywords: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Coronavirus disease 2019 (COVID-19), Spike, Receptor-binding domain (RBD), Mutation
1. Introduction
The Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses an unprecedented impact on global health and the economy. As of 7 June 2021, there have been 173,005,553 confirmed cases of COVID-19, including 3,727,605 deaths.
SARS-CoV-2 belongs to the Coronaviridae family, Betacoronavirus genus, and Sarbecovirus subgenus, containing a linear single-stranded positive-sense RNA genome of 29.9 kb (Li et al., 2020b; Lu et al., 2020; Wang et al., 2020b; Xu et al., 2020). Numerous evidence suggested that SARS-CoV-2 is closely related to the bat SARS-like-CoVs, however, the origin of the virus and its intermediate host(s) is still unclear (Li et al., 2020b; Sun et al., 2020; Zhou and Shi, 2021). The genome of SARS-CoV-2 encodes four structural proteins, including spike (S) protein, envelope (E) protein, membrane (M) protein, and nucleocapsid (N) protein, and 16 non-structural proteins (NSP1 to NSP16) (Wang et al., 2020b).
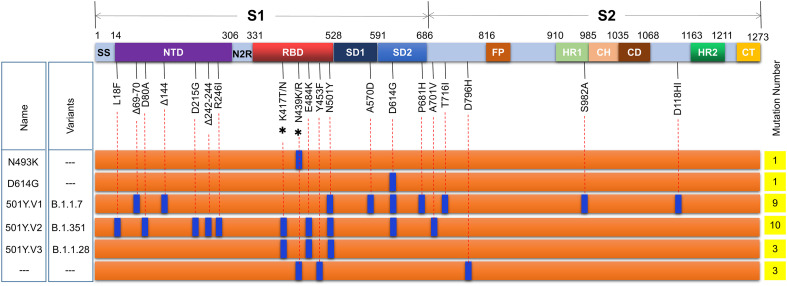
Among viral proteins, the spike protein is a glycoprotein, which anchors to the virus surface in the form of the trimer, and acts as the main antigen, and participates in the entry (Mercurio et al., 2021). SARS-CoV-2 spike harbors two cleavage sites, which are processed by proteases before membrane fusion and accelerates cell entry (Kadam et al., 2021; Seyran et al., 2020; Sun et al., 2020; Walls et al., 2020). The first cleavage site locates at the boundary between the S1 and S2 subunits (R685), which is characterized by unique polybasic furin site PRRA/R, which is absent in other known coronaviruses. The second one is at S2’ (R815) subunit, which are recognized and cleaved by transmembrane serine protease 2 (TMPRSS2) and other proteases such as cathepsin L (CPL) (Kadam et al., 2021). During the binding and entry, the S protein can be split into two subunits, S1 and S2, which facilitate affinity with cellular receptor ACE2 (Angiotensin-converting enzyme 2) and membrane fusion, respectively (Mercurio et al., 2021; Satarker and Nampoothiri, 2020). Furthermore, functional domains signal sequence (SS), NTD (N-terminal domain), RBD (receptor-binding domain), SD1 (subdomains 1), and SD2 (subdomains 2) locate in the S1 subunit, while domains FP (fusion peptide), HR1 (heptad repeat 1), CH (central helix), CD (connector domain), HR2 (heptad repeat 2), and CT (C-terminal domain) are main parts of S2 subunit (Fig. 1 ).
Fig. 1.
Major mutations in the spike protein of SARS-CoV-2. To date, more than 3698 mutations in the S protein were identified, including 2746 mutations causing amino acid changes, of which more than 340 amino acids are located in the viral RBD. Among these mutations, the most representative ones are substitution mutations such as D614G, N501Y, Y453F, N439K/R, P681H, K417N/T, and E484K, and deletion mutations of ΔH69/V70 and Δ242–244. Three mutations, D614G, N501Y, and E484K, confer the virus with enhanced infectivity, transmissibility, and resistance to neutralization. Δ, deletion; *, two meaningful mutations at this site; −--, unidentified mutations. Signal sequence (SS), NTD (N-terminal domain), N2R (NTD-to-RBD linker), RBD (receptor-binding domain), SD1 and SD2 (subdomains 1 and 2), FP (fusion peptide), HR1 (heptad repeat 1), CH (central helix), CD (connector domain), HR2 (heptad repeat 2), and CT (C-terminal domain).
The NTD (N-terminal domain) locates at 14–306 amino acids (aa) of the viral spike protein. NTD has obvious structural plasticity, involves prebinding activation and immune activation, and plays vital roles in the effective binding process and immune response together with the RBD domain (Kumar et al., 2020; Liu et al., 2020; McCallum et al.; Rosa et al., 2021). Moreover, the GTNGTKR motif at 72–78 aa of the NTD may be involved in recognizing other receptors/co-receptors besides the ACE2 (Behloul et al., 2020). Residues Y144, Y145, and V146 form a conservative pocket in the NTD of the S1 subunit of the Wuhan reference strain (GenBank No.: NC_045512), however, the deletion of amino acid residues Y144 and G107 of S protein isolated from India and France was found in the NTD, which resulted in the change of pocket structure in NTD, the decrease of affinity between NTD and endogenous monoclonal antibody, and the disruption of cell entry mediated by NTD (Dawood et al., 2021). Another report showed that a deletion of F140 in the NTD N3 loop, an insertion of 11-aa peptides (KTRNKSTSRRE) containing a glycan sequon (NKS) at N248 in the NTD N5 loop, and an E484K substitution in the RBD lead to complete resistance to plasma neutralization (Andreano et al., 2020). Besides, NTD-specific neutralizing monoclonal antibodies can inhibit cell fusion, activate effector function, and protect animals from SARS-CoV-2 challenge, avoid selecting escape mutants in animal models (McCallum et al., 2021). Therefore, mutations in the NTD may affect viral binding behavior and the binding of neutralizing antibodies.
The RBD in the S1 subunit is considered a critical domain for recognizing the ACE2 receptor and stimulating immune responses (Kadam et al., 2021; Souza et al., 2021). Similar to other SARS-CoV, the RBD in the trimer structure of SARS-CoV-2 spike protein also has two conformations: standing-up (opened conformation) and lying-down (closed conformation) (Liu et al., 2021c; Weissman et al., 2021; Yurkovetskiy et al., 2020; Zhang et al., 2020). The transitions between closed conformation and open conformation of RBD are dynamic, which are mainly caused by mutation, proteolysis, linoleic acid, or antibody binding (Kadam et al., 2021; Weissman et al., 2021; Yurkovetskiy et al., 2020; Zhang et al., 2020). During infection, the SARS-CoV-2 spike was preactivated by furin, which caused a higher proportion of RBD to stand up and bind to the ACE2 receptor, followed by the binding of the S1 subunit and ACE2, and then the membrane fusion and entry primed by the S2 subunit (Berger and Schaffitzel, 2020; Korber et al., 2020; Liu et al., 2021c; Mercurio et al., 2021; Shang et al., 2020; Turonova et al., 2020; Weissman et al., 2021; Yurkovetskiy et al., 2020; Zhang et al., 2020; Zhou et al., 2021a). Notably, compared with SARS-CoV, SARS-CoV-2 RBD, rather than the entire spike protein, has a higher ACE2 binding affinity (Shang et al., 2020). These results indicate that the lying-down RBD (closed conformation) of SARS-CoV-2 is beneficial to immune evasion, while the standing-up RBD (opened conformation) enhances effective binding between the RBD and ACE2 for efficient entry (Pierri, 2020; Turonova et al., 2020). Therefore, SARS-CoV-2 is more infectious than SARS-CoV. Besides, mounting evidence showed that the recently discovered SARS-CoV-2 is more infectious than the isolate reported in Wuhan, China in late December 2019, partly due to the conformational changes caused by the spike mutation (Gobeil et al., 2021; Kumar et al., 2020; Zhang et al., 2021).
The mutation and evolution of viruses are ubiquitous (Su et al., 2016), and the mutation of SARS-CoV-2 in the human population is also one of the hot topics in the past year. Compared with the influenza virus and HIV, the mutation rate of SARS-CoV-2 is lower (Abdelrahman et al., 2020; Rausch et al., 2020). However, due to a large number of infected people, mutations in the SARS-CoV-2 genome, especially in the NTD domain, RBD domain, SD1, and SD2 of the viral spike protein, continue to increase during the pandemic. In this review, we discussed the recent process of the mutations of SARS-CoV-2 spike protein and its influence on virus transmissibility and pathogenicity.
2. Molecular evolution of SARS-CoV-2 in the human population
2.1. General overview of SRAS-CoV-2 genetic diversity
As reported, the SARS-CoV-2 spike and its receptor-binding domain (RBD) are highly variable (Choi et al., 2020; Kemp et al., 2021; Thomson et al., 2021). As of 27 May 2021, SARS-CoV-2 can be subdivided into hundreds of lineages based on the types of mutations, among which mutations in the ORF1ab and S protein account for the largest proportion, followed by mutations in the N, ORF3a, and M protein. These results are similar to those reported by Su's group that most recombination occurred in the ORF1ab gene of porcine deltacoronavirus (PDCoV) and other human- and animal CoVs, not structural genes (He et al., 2020; Su et al., 2016). The number of nucleic acid mutation and amino acid mutation in the ORF1ab are 20,396 (frequency of nucleic acid mutation: 0.958%) and 15,091 (frequency of amino acid mutation: 2.126%), and the number of nucleic acid mutation and amino acid mutation in the spike are 3698 (frequency of nucleic acid mutation: 0.968%) and 2746 (frequency of amino acid mutation: 2.157%), respectively. Moreover, SARS-CoV-2 strains with different mutations from different patients have obvious differences in replication and infectivity (Korber et al., 2020; Thomson et al., 2021). The binding ability of the spike of the circulating variants to human ACE2 was significantly enhanced, which led to a significant increase in its replication and transmission (Korber et al., 2020; Thomson et al., 2021).
The most representative ones in the spike protein are substitution mutations such as D614G, N501Y, Y453F, N439K/R, P681H, K417N/T, and E484K, and deletion mutations of ΔH69/V70 and Δ242–244 (Fig. 1). Compared with wild-type viruses, the D614G mutation enhanced the ability of virus proliferation and transmission (Daniloski et al., 2021). The N501Y strengthened the affinity with human ACE2 and the infectivity, while 501Y·V2 variant is more resistant to multiple monoclonal antibodies, convalescent plasma, and vaccinee sera partly due to the E484K substitution (Cele et al., 2021; McCormick et al., 2021; Noh et al., 2021). Furthermore, Kemp et al. found the virus population changed dynamically during convalescent plasma therapy, which was characterized by the emergence of a dominant virus carrying double mutates in the spike, including D796H in the S2 and ΔH69/ΔV70 in the N-terminal of the S1 (Kemp et al., 2021). Although some mutations, such as D796H, may lead to a decrease in infectivity, it seems the deletions of ΔH69/ΔV70 can enhance the viral infectivity by more than two times to compensate for this decrease, and finally resulting in a moderate decrease in sensitivity to convalescent plasma (Kemp et al., 2021). The sentinel mutation N439K in the viral receptor-binding motif endows the virus with enhanced binding affinity to human ACE2, resistance to several neutralizing monoclonal antibodies, and polyclonal sera from the recovered patients (Thomson et al., 2021). A recent paper identified a novel neutrophil elastase (ELANE) cleavage site near the S1-S2 junction of the spike protein in the SARS-CoV-2614G-mutant (D614G), which may be the reason for enhancing the spread of SARS-CoV-2 in high Alpha-anti-trypsin (AAT)-deficient regions (Bhattacharyya et al., 2021). These results demonstrate that the mutation of SARS-CoV-2 will persist in the population for a long time.
During the infection, recombination and natural selection promoted the evolution and transmission of SARS-CoV-2 in humans and animals. Host immune responses induced by host gene editing, namely APOBEC (apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like) and ADAR (adenosine deaminases acting on RNA), are the main driving forces of SARS-CoV-2 evolution, accounting for nearly 65% of the recorded mutations (Wang et al., 2020d; Wu et al., 2020). Thus, under the pressure of host selections (including gene editing and immune response), SARS-CoV-2 undergoes continuous mutations in different hosts, and finally exists and spreads as a more toxic and/or infectious variant, which was prevalent in Britain, South Africa, and other countries. Furthermore, more than 1500 mutations in SARS-CoV-2 have been identified in Russia, and 18 mutations of SARS-CoV-2 were found in a Russian woman with low immunity, some of which are the same as the mutated virus appearing in Britain, and two others are consistent with the variant carried by Danish mink (Y453F and Δ69-70 HV). Especially, SARS-CoV-2 isolated from an immunocompromised person contain 57% and 38% mutations in the spike gene and its receptor-binding domain (RBD), respectively (Choi et al., 2020). These results indicate the long-term existence of SARS-CoV-2 in humans will lead to the continuous evolution of the spike by substitution in the RBD and/or deletion and insertion in N-terminal domain loops of the spike due to the relaxed selective constraint and/or positive selection, especially in the immunocompromised host, which will lead to the decrease of virus sensitivity to neutralizing antibodies and the increase of infectivity and then escape from the human immune response.
2.2. Representative variants of SARS-CoV-2 in the human population
2.2.1. D614G
From the end of January 2020, the SARS-CoV-2 D614G strain, characterized by D614G substitution in the viral spike protein, gradually replaced other subtypes and spread widely, becoming the main circulating strain of the COVID-19 pandemic (Isabel et al., 2020).
The D614G mutation leads to increased replication and transmission in primary human cells and animal models but does not affect virus virulence (Grubaugh et al., 2020; Hou et al., 2020; Isabel et al., 2020; Ozono et al., 2021; Zhou et al., 2021a). However, the exact mechanism is still controversial. Several groups found that the D614G substitution of the viral spike protein enhances the affinity with host receptor ACE2 (Ozono et al., 2021; Zhou et al., 2021a) and susceptibility to neutralization (Weissman et al., 2021), another group demonstrates the mutation does not alter spike protein binding to ACE2 (Zhang et al., 2020), while Yurkovetskiy et al. found that the affinity between D614G strain and cellular ACE2 receptor was reduced due to the faster dissociation rate (Yurkovetskiy et al., 2020). Further studies showed that D614G mutation changes the conformation of the SARS-CoV-2 spike and enhances protease cleavage at the S1/S2 junction (Gobeil et al., 2021). The amino acid D614 of the S protein forms a hydrogen bond with amino acid T859 and/or a salt bridge with amino acid K854 located in the S2 subunit, whereas amino acid G614 in the variant cannot bind to T859 and K854, providing flexible space for the S trimer (Ozono et al., 2021) and increasing furin binding (Mohammad et al., 2021), which leads to enhanced cleavage of S protein and entry of host cells. Furthermore, SARS-CoV-2 contains two identical structures in their RBD, called “3-down” and “1-up” (Weissman et al., 2021). However, the percentage of the “3-RBD-down” and “1-RBD-up” in the G614 spike (18% versus 82%) is higher than that of the parent strain D614 (54% versus 46%) (Gobeil et al., 2021; Weissman et al., 2021; Yurkovetskiy et al., 2020).
These results suggest that D614G mutation leads to allosteric effects of SD1 and SD2 domains, with a more open conformation of the S trimer, thus exposing neutralizing epitopes and ACE2 receptor binding residues on the RBD, and finally making S protein more sensitive to neutralizing antibodies and increasing the efficiency of membrane fusion.
2.2.2. N501Y
From early September 2020, a new SARS-CoV-2 variant, named 501Y Variant 1, or N501Y, was reported in United Kingdom (Leung et al., 2021; Makowski et al., 2021). The first discovered strain N501Y (501Y Variant 1) has six mutations, namely T14I, N501Y, S944L, H2357Y, P3395L, and M6723I (Leung et al., 2021). Then, a second N501Y mutant 501Y Variant 2 (also named 20B/501Y·V1, SARS-CoV-2 VOC 202012/01, or lineage B.1.1.7) appeared in England in late September 2020 and became the dominant lineage in December 2020 (Leung et al., 2021). The 501Y Variant 2 contains 17 mutations, including H69-V70 deletion (Δ69/Δ70), Y144 deletion (Δ144), N501Y, A570D, P681H, T716I, S982A, D1118H, T1001I, A1708D, I2230T, S3675-G3676-F3677 deletion, Q27stop, R52I, Y73C, D3L, and S325F (Leung et al., 2021). The N501Y strain has stronger transmission ability, which is 40–70% higher than the original strain, and the transmission index R0 of the 501Y Variant 1 and Variant 2 was 10% and 75% higher, respectively, than that of the parent strain 501 N, but the pathogenicity and reinfection rate is similar (Leung et al., 2021). Moreover, the infection rate of children has increased significantly. Thereafter, the strain soon became the main epidemic strain in London and spread in more than 50 countries in Europe and America.
Among the mutations in the B.1.1.7, nine mutations, including H69-V70 deletion (Δ69/Δ70), Y144 deletion (Δ144), N501Y, A570D, D614G, P681H, T716I, S982A, and D1119H, were located in the viral spike protein (Supasa et al., 2021). Further studies showed that the N501Y mutation significantly increases RBD:ACE2 affinity, viral entry, and infection (Leung et al., 2021; Supasa et al., 2021; Teruel et al., 2021). The H69-V70 deletion in the S1 NTD of the spike protein, which was found in SARS-CoV-2 from both human- and mink-originated SARS-CoV-2, may be associated with increased infectivity and reduced sensitivity to neutralizing antibodies (Bal et al., 2021; Kemp et al., 2021). The efficacy of non-RBD-binding monoclonal antibodies against the Δ69/Δ70 mutant was lower than that of the monoclonal antibodies targeting the RBD (Kemp et al., 2021). These results indicate the Δ69/Δ70 may induce viral escape from neutralizing antibodies.
Furthermore, a new mutation P681H was identified at the S1/S2 linkage site, which is adjacent to the furin cleavage site (682–685 aa) (Huang et al., 2020; Maison et al., 2021; Wang et al., 2020c). Numerous pieces of evidence show that the furin cleavage site promotes SARS-CoV-2 to enter respiratory epithelial cells and spread in animal models, which is considered to be the key to enhance the transmission of SARS-CoV-2. However, whether P681H affects the recognition and cleavage of S1/S2 by furin protease remains to be further studied.
These results indicate that Δ69/Δ70, N501Y, and P681H are three key mutations in the spike protein of the N501Y strain, and their effects on virus pathogenicity, transmission, and immune escape should be further evaluated. It is worth noting that N501Y mutation was detected in four SARS-CoV-2 mutants, which appeared almost simultaneously in different countries, including B.1.1.7 (UK, mid-December 2020), B.1.351 (South Africa, late December 2020), COH.20 g/N501Y (the USA, late December 2022) and P.1 strain (501Y·V3, Brazil, January 2021), suggesting that this residue may play an important role for the virus evolution and transmission.
2.2.3. 501Y·V2
In November 2020, another SARS-CoV-2 variant similar to the N501Y mutant was detected in South Africa, which was named 501Y·V2 strain (or B.1.351 lineage). Up to now, there are three most popular variants of 501Y·V2 lineage, including 501Y·V2–1, 501Y·V2–2, and 501Y·V2–3 (Li et al., 2021; Wibmer et al., 2021; Zhou et al., 2021b). The 501Y·V2–1 was the dominant variant in the early stage of the second wave of epidemic in South Africa, which enhances ACE2 affinity through seven mutations in spike protein, D614G, D80A, D215G, R246I, E484K, N501Y, and A701V. Thereafter, two other mutations L18F and K417N were identified in 501Y·V2–1, resulting in strain 501Y·V2–2. Shortly after that, an L242–244 deletion (Δ242–244) of spike protein was deleted in the 501Y·V2–2 strain, generating the third variant 501Y·V2–3 (Tegally et al., 2021; Zhou et al., 2021b).
Compared with the spike protein of SARS-CoV-2 Wuhan-1 strain, the spike protein of 501Y·V2–3 contains eight mutations: four mutations in NTD (L18F, D80A, D215G, and Δ242–244), three mutations in viral RBD (K417N, E484K, and N501Y), and one mutation in S2 region (A701V) (Li et al., 2021; Wibmer et al., 2021; Zhou et al., 2021b). Three mutations on the RBD of 501Y·V2–3 may lead to higher viral load and transmission ability than that of the Wuhan-1 strain (Li et al., 2021; Wibmer et al., 2021; Zhou et al., 2021b). Furthermore, residue K417N can bind to N501Y, thus increasing the binding between spike and ACE2 receptor in the variant (Fratev, 2020; Li et al., 2021; Wibmer et al., 2021; Zhou et al., 2021b). Mutations K417N and E484K may also reduce the sensitivity of the virus to neutralizing antibodies by more than 10 times (Li et al., 2021; Wibmer et al., 2021; Zhou et al., 2021b). Moreover, the South African variant 501Y·V2–3 can escape the inhibition of convalescent plasma and cause reinfection, which may be mainly due to the E484K and N501Y mutations (Li et al., 2021; Wibmer et al., 2021; Zhou et al., 2021b). This virus mutant, which can escape the immune system and re-infect the recovered person, has the strong advantage of becoming an epidemic strain. Moreover, to date, four mutations have been identified in amino acid 484 of spike protein, namely E484A, E484G, E484D, and E484K, and each mutation has partial resistance to the convalescent plasma, indicating that amino acid 484 is also one of the dominant epitopes of spike protein (Cele et al., 2021; Jangra et al., 2021; Li et al., 2021; Liu et al., 2021c; Wang et al., 2020e).
3. Current concerns and suggestions on SARS-CoV-2 mutation
3.1. Cross-species transmission of SARS-CoV-2 between animals and humans
Till now, although several groups speculated that SARS-CoV-2 originated from bats or pangolins, the origin of the virus and its intermediate host(s) remained a mystery. As reported, the spike protein of SARS-CoV-2 can interact with ACE2 of a variety of species in vitro and in vivo (Damas et al., 2020; Liu et al., 2021b; Wu et al., 2020; Zhai et al., 2020), suggesting that SARS-CoV-2 has the ability of cross-species recognition. The virus has also been detected in numerous non-human species by natural infection or artificial inoculation, including bat, hamster, raccoon dog, ferret, mink, monkey, rhesus macaque, gorilla, pangolin, tiger, lion, cat, dog, etc. (Bosco-Lauth et al., 2020; Kiros et al., 2020; Schlottau et al., 2020; Segales et al., 2020; Wu et al., 2020; Zhai et al., 2020), which have frequent contact with humans. Moreover, reports from Denmark and other countries confirmed that SARS-CoV-2 can be transmitted from humans to mink and raccoon dogs, and vice versa (Freuling et al., 2020; Oude Munnink et al., 2021). These results indicate the host spectrum of SARS-CoV-2 is wider than we expected, and the possibility of SARS-CoV-2 spreading between humans and animals, especially domestic or economic animals, is very high by direct contact or aerosols. Besides, SARS-CoV-2 was stable on various surfaces, including plastic, stainless steel, skin, paper, glass, etc., for a long time, especially at low temperature or cold-chain transportation (Harbourt et al., 2020; Ji et al., 2021). Thus, with the movement of people and the contact between people and animals, the spillover events of SARS-CoV-2 from animals to humans (Zhou and Shi, 2021), especially wild mammals, are inevitable.
It is worth noting that most of the SARS-CoV-2-infected animals show asymptomatic to mild symptoms, which may often be ignored by farmers or owners, resulting in a widespread of the virus. Meanwhile, existing evidence shows that SARS-CoV-2 may have originated from bat coronavirus 40–70 years ago (Chaw et al., 2020; Forni et al., 2017; He and Chen, 2020), and SARS-CoV-2 appeared in Europe and America earlier than it was reported in Wuhan, China, which further confirms that SARS-CoV-2 formed or appeared in animals earlier than it appeared in humans and caused an outbreak. And we are sure that mutations of SARS-CoV-2 continue in animals and humans, although little progress has been reported on evaluating the recombinant rate of SARS-CoV-2 in animals. Therefore, continuous transmission, especially asymptomatic infection and superspreading events, will lead to many animals carrying the virus, which will transfer to more species and humans. Although experts predict that SARS-CoV-2 may become similar to other human coronaviruses causing the common cold and coexist with humans (Lavine et al., 2021), it will take a long time for the virus and humans to adapt to each other. Thus, monitoring SARS-CoV-2 in susceptible animals is still necessary before effective vaccines are widely used. People who contact animals should take necessary precautions, the hunting and consumption of wild animals should be permanently banned. More attention needs to be paid to the transmission of SARS-CoV-2 and its variants between humans and animals.
3.2. SARS-CoV-2 variants affect the efficacy of the vaccine and therapeutic antibodies
It was reported that the human sera from people immunized with Pfizer BTN162b2 vaccine can neutralize three SARS-CoV-2 viruses containing key spike mutations, including N501Y, 69/70-deletion+N501Y + D614G, and E484K + N501Y + D614G, with neutralization geometric mean titers of 0.81–1.46 times of the parental virus (Xie et al., 2021). Furthermore, other vaccines, including BBIBP-CorV (Sinopharm) and CoronaVac (Sinovac) in China, mRNA-1273 vaccine (Moderna) in the United States, Sputnik-V vaccine in Russia, most of which are aimed at the viral spike or its RBD, have been granted emergency use authorization. However, the protective rate and effectiveness of each vaccine to different variants are distinct. It is unclear whether these vaccines are still effective against SARS-CoV-2 mutants continuously generated in the population because increasing evidence shows that SARS-CoV-2 Variants B.1.351 and B.1.1.7 are resistant to neutralizing antibodies in convalescent plasma and vaccinee sera (Chen et al., 2021; Wang et al., 2021a). Moreover, although inactivated vaccine contains all the viral components, it is not clear whether the inactivation process will affect the activity of the spike and the integrity of the virus. Besides, antibodies, such as Regeneron's REGN-COV2 cocktail (REGN10933 and REGN10987) and Eli Lilly's LY-CoV016 antibody can effectively neutralize the virus, however, viral mutations in the circulating strains can weaken the recognition mediated by the antibodies and polyclonal human serum (Andreano et al., 2020; Starr et al., 2021). These results suggest SARS-CoV-2 may escape human immune response via continuous genomic evolution by substitution in the viral RBD and/or deletion and insertion in N-terminal domain loops of the viral spike, especially in the immunocompromised host (Andreano et al., 2020; Clark et al., 2021; Starr et al., 2021). We previously also found pseudorabies virus can escape the inhibition mediated by CRISPR-Cas9 targeting a single site by substitution (Peng et al., 2016). Therefore, according to the experience of vaccines (such as influenza and HPV vaccines) that have been used in the form of multivalent vaccines, it is necessary to evaluate the effectiveness of current vaccines against SARS-CoV-2 variants, and update vaccines and therapeutic antibodies in time according to virus mutations.
As reported, the S2 subunit, RBD, NTD, M protein, and E protein of SARS-CoV2 are effective target sites for developing the suitable vaccine for COVID-19 by comparing the genomes of various coronavirus strains (Kaur et al., 2021). A recent report showed that bispecific IgG based on two antibodies from COVID-19 convalescent donors can effectively neutralize SARS-CoV-2 and its variants, and prevent the animal from disease and inhibit viral escape in mice models (De Gasparo et al., 2021). Moreover, mosaic-RBD-nanoparticles elicited robust antibodies with superior cross-reactive recognition of heterologous RBDs than that of homotypic nanoparticles or COVID-19 convalescent human plasmas (Cohen et al., 2021). The neutralization activity of multivalent nanoparticles targeting different epitopes on the RBD is more than 100 times higher than that of monovalent nanoparticles (Koenig et al., 2021). The escape-resistant antibody cocktails, including antibody cocktails that compete to bind to the same RBD surface but have different escape mutations, are effective candidates for therapeutic cocktails (Greaney et al., 2021). Several nanoparticles neutralized SARS-CoV-2 via receptor binding competition, while other monovalent and bivalent nanoparticles trigger the abnormal activation of spike fusion (Koenig et al., 2021). These results indicate the heterologous multi-target vaccines and cocktail antibodies can effectively inhibit SARS-CoV-2 infection, which are promising vaccines and therapeutic agents against SARS-CoV-2 and its mutants. Therefore, apart from the monovalent vaccine, we strongly recommend developing multivalent vaccines and cocktail antibodies targeting different epitopes of the RBD and conserved viral components, such as nucleocapsid, envelope, and membrane proteins, as soon as possible to provide cross-reactive immunity and control the pandemic.
3.3. Beneficial intestinal microbes can be used to alleviate severe symptoms and improve the prognosis of COVID-19
The gut microbiome plays important role in modulating host immune response and affecting viral pathogenesis and secondary infection in the gastrointestinal (GI) tract. As reported, patients infected with SARS-CoV-2 show a wide range of GI disorders, including anorexia, nausea, vomiting, and abdominal pain (Luo et al., 2021). The gut microbiome is also associated with the COVID-19 severity and levels of plasma concentrations of cytokines, chemokines, and inflammation markers (Luo et al., 2021; Yeoh et al., 2021). The composition of gut microflora in patients with COVID-19 and even recovered patients is unbalanced, which is characterized by the decrease of beneficial microbes and the increase of harmful ones (Yeoh et al., 2021). These results suggest that the GI disorders caused by COVID-19 are mainly due to the destruction of intestinal mechanical barrier integrity, alteration of the intestinal microflora, and systemic inflammatory response to the virus (Luo et al., 2021; Syed et al., 2020; Villapol, 2020). Therefore, it is recommended to properly add beneficial intestinal microbes, especially probiotics and prebiotics, which may enhance the intestinal barrier function, help to improve and alleviate the sequela of COVID-19 and accelerate the complete recovery of patients. The beneficial intestinal microflora and good intestinal barrier may be helpful for the human body to resist SARS-Cov-2, while the imbalance of intestinal microflora may lead to an increased risk and adverse results of the COVID-19.
During infection, SARS-CoV-2 interacts with at least four confirmed host receptors or co-receptors, including ACE2, transmembrane serine protease 2 (TMPRSS2), neuropilin-1 (NRP1), and tyrosine-protein kinase receptor UFO (AXL), which either mediate the viral infection alone or act synergistically with each other (Cantuti-Castelvetri et al., 2020; Daly et al., 2020; Hoffmann et al., 2020; Nie et al., 2021; Wang et al., 2021a; Wang et al., 2021b; Wang et al., 2020e). High expression of ACE2 was detected in the nasal goblet and ciliated cells, duodenum, small intestine, gallbladder, kidney, and testis, while high expression of TMPRSS2 was observed in the kidney, parathyroid gland, stomach, pancreas, epididymis, and prostate (Wang et al., 2020e). Notably, the number of tissues and organs with high NRP1 expression was more than those with high ACE2 and TMPRSS2 expression (Wang et al., 2020e). Therefore, SARS-CoV-2 can infect almost all human organs, including the respiratory tract, digestive system organs, heart, kidney, brain, bladder, liver, cornea/conjunctiva, lymph nodes, and/or reproductive organs, and cause multiple organ damages, among which nasal and intestine are the first to encounter the virus and produce mucosal immunity (Singh et al., 2020; Villena et al., 2021). It is worth noting that IgG specific to the receptor-binding domain of the viral spike is highly correlated with neutralizing antibodies for the first few months (3 to 5 months) after SARS-CoV-2 infection (Iyer et al., 2020). Most patients with mild-to-moderate symptoms have a strong humoral response to the spike, and the relatively stable IgG lasts at least 5 months after infection (Iyer et al., 2020). However, IgM and IgA against the viral spike decayed in 2.5 months after the onset of the disease (Iyer et al., 2020). These results indicate that strengthening the mucosal immune response may be beneficial to the treatment of the disease.
Lactic acid bacteria are food-grade probiotics, which are isolated from the nasal and intestine of humans or animals. These bacteria have high intestinal adhesion and strong anti-inflammatory and immunomodulatory functions (Villena et al., 2021; Wang et al., 2020a). During the last decades, lactic acid bacteria have been widely used to express and display target antigens in nasal and oral vaccines. Mounting evidence showed that these vaccines can induce a significant immune response, especially mucosal immune response, provide efficient protection against infection, and improve lung-gut axis health by nasal or oral administration. Altered gut microbiota has been observed in COVID-19 patients leading to an enrichment of opportunistic pathogens and a depletion of beneficial bacteria (Wang et al., 2020a). We previously constructed a recombinant Lactobacillus plantarum expressing the spike of SARS-CoV-2 (Wang et al., 2020a). The recombinant bacterium can induce high levels of effective mucosal immune response via intranasal or oral administration (Villena et al., 2021; Wang et al., 2020a), which indicates that it is a promising vaccine for the COVID-19 pandemic. The efficiency and protective rate of the vaccine are still under evaluation.
Notably, there is no significant difference in the prognosis of COVID-19 patients with or without antibiotics (Moretto et al., 2021; Yeoh et al., 2021), suggesting antibiotics do not affect the treatment but can aggravate the imbalance of intestinal flora. Therefore, the use of antibiotics should be carefully considered or restricted in the treatment of the COVID-19.
3.4. Intranasal vaccine is a promising candidate vaccine to prevent the infection and spread of SARS-CoV -2
A recent paper published in PNAS showed one or two injections of modified vaccinia virus-vectored vaccine could effectively protect hACE2 transgenic mice against lethal infection of SARS-CoV-2 in the upper and lower respiratory tract (Liu et al., 2021a). The vaccine can induce spike-specific CD3 + CD8 + IFNγ+ T cells and reduce cytokine and chemokine profiles (Liu et al., 2021a). Furthermore, to date, there are more than 150 candidate vaccines at various stages of development, especially several vaccines that were granted emergency use authorizations, such as BBIBP-CorV (Sinopharm) and CoronaVac (Sinovac) in China, Pfizer-BioNTech COVID-19 vaccine (Pfizer), and mRNA-1273 vaccine (Moderna) in the United States, Sputnik-V vaccine in Russia. The results suggest the vaccines targeted to the viral spike or its receptor-binding domain (RBD) are effective for inducing an immune response against SARS-CoV-2. However, most of these vaccines are administered by intramuscular injection in two or more doses, which can stimulate robust humoral and cellular immune responses against infection, but does not confer mucosal immunity and eradicate the virus.
SARS-CoV-2 is mainly transmitted through the respiratory system by interacting with host receptors or co-receptors, including ACE2, TMPRSS2, etc. High expression of ACE2 was detected in the nasal goblet and ciliated cells, duodenum, small intestine, gallbladder, kidney, and testis, while high levels of TMPRSS2 were observed in the kidney, parathyroid gland, stomach, pancreas, epididymis, and prostate. Therefore, SARS-CoV-2 can infect almost all human organs, among which the nasal and intestine are the first barrier to encounter the virus (Singh et al., 2020; Villena et al., 2021).
Notably, most patients with mild-to-moderate symptoms have a strong humoral response to the spike, and the IgG lasts at least 5 months after infection. The early SARS-CoV-2-specific humoral responses were dominated by IgA, especially IgA dimers in the nasopharynx (Wang et al., 2021c). However, IgM and IgA decayed in 2.5 months after the onset of the disease. Thus, strengthening the mucosal immune response may be beneficial to the treatment of the disease. We constructed a recombinant Lactobacillus plantarum, which can efficiently display the viral spike on the bacterial surface and induce high levels of effective mucosal immune response via intranasal or oral administration (Wang et al., 2020a). The efficiency and protective rate of the vaccine are still under evaluation. Moreover, a single dose of intranasal chimpanzee adenovirus-vectored vaccine encoding the spike (ChAd-SARS-CoV-2-S) or intranasal adenovirus type 5-vectored vaccine targeting the RBD (AdCOVID) can entirely prevent SARS-CoV-2 infection in both the upper and lower respiratory tracts of animal models, with high levels of serum-neutralizing antibodies, systemic and mucosal IgA, and CD4+ and CD8+ T cells with Th1-like cytokine expressions in the respiratory tracts (Hassan et al., 2020; King et al., 2020). Meanwhile, CD8+ T cells play a front-line role in the fight against respiratory virus infection, and nasal mucosal immunity is a local immunity that effectively inhibits respiratory virus infection and transmission. It is predicted SARS-CoV-2 may become similar to other human coronaviruses causing the common cold and coexist with humans. Therefore, same as the nasal flu vaccine for seasonal influenza virus, the intranasal vaccines may be promising candidate vaccines preventing SARS-CoV-2 infection and transmission, which can rapidly cover the whole-body mucosa within 3–5 days.
4. Conclusion and perspective
Since the outbreak of the COVID-19 pandemic, various studies on SARS-CoV-2 have been carried out rapidly and comprehensively. Basic research has been deepened, relevant diagnostic methods and treatment measures have been gradually improved (Li and Ren, 2020; Li et al., 2020a), and several vaccines have been granted emergency authorization. However, the understanding of basic biological problems in SARS-CoV-2 is still very limited, such as the details of the virus life cycle and the molecular mechanism of interaction with the host. Especially, the variation of the virus in the process of natural evolution or under the pressure of host immunity has a great influence on its pathogenicity, transmission ability, and antibody neutralization level (Fig. 2 ). Therefore, it is necessary to continuously monitor the variation of the virus genome, especially the amino acid variation of the spike, and analyze and predict the dominant virus strains for the development of vaccine and antiviral agents. Furthermore, whether the vaccines used in the clinic or being developed can provide effective protection against the variants with continuous mutation of the genome, whether the different virus subtypes and mutants produced by the mutation have cross or dominant epitopes, cross-species infection potential, and ability to evade immune recognition, etc. are the major key scientific issues that need further exploration.
Fig. 2.
Possible ways to enhance the spread of SARS-CoV-2. SARS-CoV-2 is transmitted among different hosts during the pandemic via direct contact, aerosols, or the virus-contaminated environment. During the infection, the virus interacts with various cellular receptors, which either mediate the viral infection alone or act synergistically with each other. Furthermore, recombination and natural selection promote the evolution and transmission of SARS-CoV-2 in humans and animals. Subsequently, SARS-CoV-2 coexists with human beings or animals.
Besides, with the continuation of the pandemic in COVID-19, it is of great significance to understand the factors that may promote virus mutation and their influence on virus behavior. Meanwhile, the analysis of the above problems will accelerate the more effective drug screening and vaccine development for SARS-CoV-2, and will also provide a very powerful theoretical basis for the prevention and control of the COVID-19 pandemic.
Author contributions
Conceptualization, R.L. and L.C.; writing-original draft preparation, L.X., and Z.L.; writing-review and revision, R.L., J.W., and C.S.; figure, R.L. and L.C.; supervision, L.R.; and funding acquisition, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China [Grant No.: 31772747], the Jilin Province Science and Technology Development Projects [Grant No.: 20200402043NC]. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability statement
Not applicable.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- Abdelrahman Z., Li M., Wang X. Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza a respiratory viruses. Front. Immunol. 2020;11:552909. doi: 10.3389/fimmu.2020.552909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano E., Piccini G., Licastro D., Casalino L., Johnson N.V., Paciello I., Monego S.D., Pantano E., Manganaro N., Manenti A., Manna R., Casa E., Hyseni I., Benincasa L., Montomoli E., Amaro R.E., McLellan J.S., Rappuoli R. bioRxiv. 2020. SARS-CoV-2 escape in vitro from a highly neutralizing COVID-19 convalescent plasma. 2020.2012.2028.424451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal A., Destras G., Gaymard A., Stefic K., Marlet J., Eymieux S., Regue H., Semanas Q., d’Aubarede C., Billaud G., Laurent F., Gonzalez C., Mekki Y., Valette M., Bouscambert M., Gaudy-Graffin C., Lina B., Morfin F., Josset L., Group, C.O.-D.H.S Two-step strategy for the identification of SARS-CoV-2 variant of concern 202012/01 and other variants with spike deletion H69-V70, France, august to December 2020. Euro. Surveill. 2021;26:2100008. doi: 10.2807/1560-7917.ES.2021.26.3.2100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behloul N., Baha S., Shi R., Meng J. Role of the GTNGTKR motif in the N-terminal receptor-binding domain of the SARS-CoV-2 spike protein. Virus Res. 2020;286:198058. doi: 10.1016/j.virusres.2020.198058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger I., Schaffitzel C. The SARS-CoV-2 spike protein: balancing stability and infectivity. Cell Res. 2020;30:1059–1060. doi: 10.1038/s41422-020-00430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya C., Das C., Ghosh A., Singh A.K., Mukherjee S., Majumder P.P., Basu A., Biswas N.K. SARS-CoV-2 mutation 614G creates an elastase cleavage site enhancing its spread in high AAT-deficient regions. Infect. Genet. Evol. 2021;90:104760. doi: 10.1016/j.meegid.2021.104760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco-Lauth A.M., Hartwig A.E., Porter S.M., Gordy P.W., Nehring M., Byas A.D., VandeWoude S., Ragan I.K., Maison R.M., Bowen R.A. Experimental infection of domestic dogs and cats with SARS-CoV-2: pathogenesis, transmission, and response to reexposure in cats. Proc. Natl. Acad. Sci. U. S. A. 2020;117:26382–26388. doi: 10.1073/pnas.2013102117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Osterlund P., Joensuu M., Meunier F.A., Butcher S.J., Winkler M.S., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cele S., Gazy I., Jackson L., Hwa S.H., Tegally H., Lustig G., Giandhari J., Pillay S., Wilkinson E., Naidoo Y., Karim F., Ganga Y., Khan K., Bernstein M., Balazs A.B., Gosnell B.I., Hanekom W., Moosa M.S., Network for Genomic Surveillance in South, A, Team C.-K., Lessells R.J., de Oliveira T., Sigal A. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021;593:142–146. doi: 10.1038/s41586-021-03471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaw S.M., Tai J.H., Chen S.L., Hsieh C.H., Chang S.Y., Yeh S.H., Yang W.S., Chen P.J., Wang H.Y. The origin and underlying driving forces of the SARS-CoV-2 outbreak. J. Biomed. Sci. 2020;27:73. doi: 10.1186/s12929-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B., Choudhary M.C., Regan J., Sparks J.A., Padera R.F., Qiu X., Solomon I.H., Kuo H.H., Boucau J., Bowman K., Adhikari U.D., Winkler M.L., Mueller A.A., Hsu T.Y., Desjardins M., Baden L.R., Chan B.T., Walker B.D., Lichterfeld M., Brigl M., Kwon D.S., Kanjilal S., Richardson E.T., Jonsson A.H., Alter G., Barczak A.K., Hanage W.P., Yu X.G., Gaiha G.D., Seaman M.S., Cernadas M., Li J.Z. Persistence and evolution of SARS-CoV-2 in an Immunocompromised host. N. Engl. J. Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S.A., Clark L.E., Pan J., Coscia A., McKay L.G.A., Shankar S., Johnson R.I., Brusic V., Choudhary M.C., Regan J., Li J.Z., Griffiths A., Abraham J. SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms. Cell. 2021;184(2605–2617) doi: 10.1016/j.cell.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.A., Gnanapragasam P.N.P., Lee Y.E., Hoffman P.R., Ou S., Kakutani L.M., Keeffe J.R., Wu H.J., Howarth M., West A.P., Barnes C.O., Nussenzweig M.C., Bjorkman P.J. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science. 2021;371:735–741. doi: 10.1126/science.abf6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.L., Simonetti B., Klein K., Chen K.E., Williamson M.K., Anton-Plagaro C., Shoemark D.K., Simon-Gracia L., Bauer M., Hollandi R., Greber U.F., Horvath P., Sessions R.B., Helenius A., Hiscox J.A., Teesalu T., Matthews D.A., Davidson A.D., Collins B.M., Cullen P.J., Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas J., Hughes G.M., Keough K.C., Painter C.A., Persky N.S., Corbo M., Hiller M., Koepfli K.P., Pfenning A.R., Zhao H., Genereux D.P., Swofford R., Pollard K.S., Ryder O.A., Nweeia M.T., Lindblad-Toh K., Teeling E.C., Karlsson E.K., Lewin H.A. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc. Natl. Acad. Sci. U. S. A. 2020;117:22311–22322. doi: 10.1073/pnas.2010146117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniloski Z., Jordan T.X., Ilmain J.K., Guo X., Bhabha G., tenOever B.R., Sanjana N.E. The spike D614G mutation increases SARS-CoV-2 infection of multiple human cell types. Elife. 2021;10 doi: 10.7554/eLife.65365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood R.M., El-Meguid M.A., Salum G.M., El-Wakeel K., Shemis M., El Awady M.K. Bioinformatics prediction of B and T cell epitopes within the spike and nucleocapsid proteins of SARS-CoV2. J. Infect. Public Health. 2021;14:169–178. doi: 10.1016/j.jiph.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gasparo R., Pedotti M., Simonelli L., Nickl P., Muecksch F., Cassaniti I., Percivalle E., Lorenzi J.C.C., Mazzola F., Magri D., Michalcikova T., Haviernik J., Honig V., Mrazkova B., Polakova N., Fortova A., Tureckova J., Iatsiuk V., Di Girolamo S., Palus M., Zudova D., Bednar P., Bukova I., Bianchini F., Mehn D., Nencka R., Strakova P., Pavlis O., Rozman J., Gioria S., Sammartino J.C., Giardina F., Gaiarsa S., Pan-Hammarstrom Q., Barnes C.O., Bjorkman P.J., Calzolai L., Piralla A., Baldanti F., Nussenzweig M.C., Bieniasz P.D., Hatziioannou T., Prochazka J., Sedlacek R., Robbiani D.F., Ruzek D., Varani L. Bispecific IgG neutralizes SARS-CoV-2 variants and prevents escape in mice. Nature. 2021;593:424–428. doi: 10.1038/s41586-021-03461-y. [DOI] [PubMed] [Google Scholar]
- Chen R.E., Zhang X., Case J.B., Winkler E.S., Liu Y., VanBlargan L.A., Liu J., Errico J.M., Xie X., Suryadevara N., Gilchuk P., Zost S.J., Tahan S., Droit L., Turner J.S., Kim W., Schmitz A.J., Thapa M., Wang D., Boon A.C.M., Presti R.M., O'Halloran J.A., Kim A.H.J., Deepak P., Pinto D., Fremont D.H., Crowe J.E., Corti D., Virgin H.W., Ellebedy A.H., Shi P.Y., Diamond M.S. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021;27(4):717–726. doi: 10.1038/s41591-021-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni D., Cagliani R., Clerici M., Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25:35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratev F. The N501Y and K417N mutations in the spike protein of SARS-CoV-2 alter the interactions with both hACE2 and human derived antibody: a free energy of perturbation study. bioRxiv. 2020 doi: 10.1101/2020.12.23.424283. 2020.2012.2023.424283. [DOI] [PubMed] [Google Scholar]
- Freuling C.M., Breithaupt A., Muller T., Sehl J., Balkema-Buschmann A., Rissmann M., Klein A., Wylezich C., Hoper D., Wernike K., Aebischer A., Hoffmann D., Friedrichs V., Dorhoi A., Groschup M.H., Beer M., Mettenleiter T.C. Susceptibility of raccoon dogs for experimental SARS-CoV-2 infection. Emerg. Infect. Dis. 2020;26:2982–2985. doi: 10.3201/eid2612.203733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil S.M., Janowska K., McDowell S., Mansouri K., Parks R., Manne K., Stalls V., Kopp M.F., Henderson R., Edwards R.J., Haynes B.F., Acharya P. D614G mutation alters SARS-CoV-2 spike conformation and enhances protease cleavage at the S1/S2 junction. Cell Rep. 2021;34:108630. doi: 10.1016/j.celrep.2020.108630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A.J., Starr T.N., Gilchuk P., Zost S.J., Binshtein E., Loes A.N., Hilton S.K., Huddleston J., Eguia R., Crawford K.H.D., Dingens A.S., Nargi R.S., Sutton R.E., Suryadevara N., Rothlauf P.W., Liu Z., Whelan S.P.J., Carnahan R.H., Crowe J.E., Jr., Bloom J.D. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29(44–57) doi: 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubaugh N.D., Hanage W.P., Rasmussen A.L. Making sense of mutation: what D614G means for the COVID-19 pandemic remains unclear. Cell. 2020;182:794–795. doi: 10.1016/j.cell.2020.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbourt D.E., Haddow A.D., Piper A.E., Bloomfield H., Kearney B.J., Fetterer D., Gibson K., Minogue T. Modeling the stability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on skin, currency, and clothing. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., Chen R.E., Winkler E.S., Wessel A.W., Case J.B., Kashentseva E., McCune B.T., Bailey A.L., Zhao H., VanBlargan L.A., Dai Y.N., Ma M., Adams L.J., Shrihari S., Danis J.E., Gralinski L.E., Hou Y.J., Schafer A., Kim A.S., Keeler S.P., Weiskopf D., Baric R.S., Holtzman M.J., Fremont D.H., Curiel D.T., Diamond M.S. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(169–184) doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Chen Q. Progress in source tracking of SARS-CoV-2. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40:1838–1842. doi: 10.12122/j.issn.1673-4254.2020.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W.T., Ji X., He W., Dellicour S., Wang S., Li G., Zhang L., Gilbert M., Zhu H., Xing G., Veit M., Huang Z., Han G.Z., Huang Y., Suchard M.A., Baele G., Lemey P., Su S. Genomic epidemiology, evolution, and transmission dynamics of porcine Deltacoronavirus. Mol. Biol. Evol. 2020;37:2641–2654. doi: 10.1093/molbev/msaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(271–280) doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.J., Chiba S., Halfmann P., Ehre C., Kuroda M., Dinnon K.H., 3rd, Leist S.R., Schafer A., Nakajima N., Takahashi K., Lee R.E., Mascenik T.M., Graham R., Edwards C.E., Tse L.V., Okuda K., Markmann A.J., Bartelt L., de Silva A., Margolis D.M., Boucher R.C., Randell S.H., Suzuki T., Gralinski L.E., Kawaoka Y., Baric R.S. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370:1464–1468. doi: 10.1126/science.abe8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Yang C., Xu X.F., Xu W., Liu S.W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabel S., Grana-Miraglia L., Gutierrez J.M., Bundalovic-Torma C., Groves H.E., Isabel M.R., Eshaghi A., Patel S.N., Gubbay J.B., Poutanen T., Guttman D.S., Poutanen S.M. Evolutionary and structural analyses of SARS-CoV-2 D614G spike protein mutation now documented worldwide. Sci. Rep. 2020;10:14031. doi: 10.1038/s41598-020-70827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., Mills R., Teng E., Kamruzzaman M., Garcia-Beltran W.F., Astudillo M., Yang D., Miller T.E., Oliver E., Fischinger S., Atyeo C., Iafrate A.J., Calderwood S.B., Lauer S.A., Yu J., Li Z., Feldman J., Hauser B.M., Caradonna T.M., Branda J.A., Turbett S.E., LaRocque R.C., Mellon G., Barouch D.H., Schmidt A.G., Azman A.S., Alter G., Ryan E.T., Harris J.B., Charles R.C. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangra S., Ye C., Rathnasinghe R., Stadlbauer D., Personalized Virology Initiative study, g, Krammer F., Simon V., Martinez-Sobrido L., Garcia-Sastre A., Schotsaert M. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe. 2021 doi: 10.1016/S2666-5247(21)00068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Li X., Chen S., Ren L. Transmission of SARS-CoV-2 via fomite, especially cold chain, should not be ignored. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2026093118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S.B., Sukhramani G.S., Bishnoi P., Pable A.A., Barvkar V.T. SARS-CoV-2, the pandemic coronavirus: molecular and structural insights. J. Basic Microbiol. 2021;61:180–202. doi: 10.1002/jobm.202000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur N., Singh R., Dar Z., Bijarnia R.K., Dhingra N., Kaur T. Genetic comparison among various coronavirus strains for the identification of potential vaccine targets of SARS-CoV2. Infect. Genet. Evol. 2021;89:104490. doi: 10.1016/j.meegid.2020.104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp S.A., Collier D.A., Datir R.P., Ferreira I., Gayed S., Jahun A., Hosmillo M., Rees-Spear C., Mlcochova P., Lumb I.U., Roberts D.J., Chandra A., Temperton N., Consortium, C.-G.U, Sharrocks K., Blane E., Modis Y., Leigh K.E., Briggs J.A.G., van Gils M.J., Smith K.G.C., Bradley J.R., Smith C., Doffinger R., Ceron-Gutierrez L., Barcenas-Morales G., Pollock D.D., Goldstein R.A., Smielewska A., Skittrall J.P., Gouliouris T., Goodfellow I.G., Gkrania-Klotsas E., Illingworth C.J.R., McCoy L.E., Gupta R.K., Collaboration, C.-N.B.C SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021;592:277–282. doi: 10.1038/s41586-021-03291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R.G., Silva-Sanchez A., Peel J.N., Botta D., Meza-Perez S., Allie R., Schultz M.D., Liu M., Bradley J.E., Qiu S., Yang G., Zhou F., Zumaquero E., Simpler T.S., Mousseau B., Killian J.T., Dean B., Shang Q., Tipper J.L., Risley C., Harrod K.S., Feng R., Lee Y., Shiberu B., Krishnan V., Peguillet I., Zhang J., Green T., Randall T.D., Georges B., Lund F.E., Roberts S. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 in mice. bioRxiv. 2020 doi: 10.1101/2020.10.10.331348. 2020.2010.2010.331348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiros M., Andualem H., Kiros T., Hailemichael W., Getu S., Geteneh A., Alemu D., Abegaz W.E. COVID-19 pandemic: current knowledge about the role of pets and other animals in disease transmission. Virol. J. 2020;17:143. doi: 10.1186/s12985-020-01416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig P.A., Das H., Liu H., Kummerer B.M., Gohr F.N., Jenster L.M., Schiffelers L.D.J., Tesfamariam Y.M., Uchima M., Wuerth J.D., Gatterdam K., Ruetalo N., Christensen M.H., Fandrey C.I., Normann S., Todtmann J.M.P., Pritzl S., Hanke L., Boos J., Yuan M., Zhu X., Schmid-Burgk J.L., Kato H., Schindler M., Wilson I.A., Geyer M., Ludwig K.U., Hallberg B.M., Wu N.C., Schmidt F.I. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science. 2021;371 doi: 10.1126/science.abe6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., Sheffield C.-G.G., McDanal C., Perez L.G., Tang H., Moon-Walker A., Whelan S.P., LaBranche C.C., Saphire E.O., Montefiori D.C. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(812–827) doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.G., Ogden D.S., Isu U.H., Polasa A., Losey J., Moradi M. Differential dynamic behavior of prefusion spike proteins of SARS coronaviruses 1 and 2. bioRxiv. 2020 doi: 10.1101/2020.12.25.424008. 2020.2012.2025.424008. [DOI] [Google Scholar]
- Lavine J.S., Bjornstad O.N., Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021;371:741–745. doi: 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K., Shum M.H., Leung G.M., Lam T.T., Wu J.T. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro. Surveill. 2021;26:2002106. doi: 10.2807/1560-7917.ES.2020.26.1.2002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ren L. Recent progress on the diagnosis of 2019 novel coronavirus. Transbound. Emerg. Dis. 2020;67:1485–1491. doi: 10.1111/tbed.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang L., Ren L. Antiviral mechanisms of candidate chemical medicines and traditional Chinese medicines for SARS-CoV-2 infection. Virus Res. 2020;286:198073. doi: 10.1016/j.virusres.2020.198073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Yang Y., Ren L. Genetic evolution analysis of 2019 novel coronavirus and coronavirus from other species. Infect. Genet. Evol. 2020;82:104285. doi: 10.1016/j.meegid.2020.104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Nie J., Wu J., Zhang L., Ding R., Wang H., Zhang Y., Li T., Liu S., Zhang M., Zhao C., Liu H., Nie L., Qin H., Wang M., Lu Q., Li X., Liu J., Liang H., Shi Y., Shen Y., Xie L., Zhang L., Qu X., Xu W., Huang W., Wang Y. SARS-CoV-2 501Y.V2 variants lack higher infectivity but do have immune escape. Cell. 2021;184 doi: 10.1016/j.cell.2021.02.042. 2362–2371 e2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F., Sahi V., Figueroa A., Guo X.V., Cerutti G., Bimela J., Gorman J., Zhou T., Chen Z., Yuen K.Y., Kwong P.D., Sodroski J.G., Yin M.T., Sheng Z., Huang Y., Shapiro L., Ho D.D. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- Liu R., Americo J.L., Cotter C.A., Earl P.L., Erez N., Peng C., Moss B. One or two injections of MVA-vectored vaccine shields hACE2 transgenic mice from SARS-CoV-2 upper and lower respiratory tract infection. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2026785118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Hu G., Wang Y., Ren W., Zhao X., Ji F., Zhu Y., Feng F., Gong M., Ju X., Zhu Y., Cai X., Lan J., Guo J., Xie M., Dong L., Zhu Z., Na J., Wu J., Lan X., Xie Y., Wang X., Yuan Z., Zhang R., Ding Q. Functional and genetic analysis of viral receptor ACE2 orthologs reveals a broad potential host range of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2025373118. 2020.2004.2022.046565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., VanBlargan L.A., Bloyet L.M., Rothlauf P.W., Chen R.E., Stumpf S., Zhao H., Errico J.M., Theel E.S., Liebeskind M.J., Alford B., Buchser W.J., Ellebedy A.H., Fremont D.H., Diamond M.S., Whelan S.P.J. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29(477–488) doi: 10.1016/j.chom.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Liang S., Jin F. Gut microbiota in antiviral strategy from bats to humans: a missing link in COVID-19. Sci. China Life Sci. 2021;64:942–956. doi: 10.1007/s11427-020-1847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison D.P., Ching L.L., Shikuma C.M., Nerurkar V.R. Genetic characteristics and phylogeny of 969-bp S gene sequence of SARS-CoV-2 from Hawaii reveals the worldwide emerging P681H mutation. Hawaii J Health Soc Welf. 2021;80(3):52–61. [PMC free article] [PubMed] [Google Scholar]
- Makowski L., Olson-Sidford W., Weisel J.W. Biological and clinical consequences of integrin binding via a rogue RGD motif in the SARS CoV-2 spike protein. Viruses. 2021;13:146. doi: 10.3390/v13020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum M., De Marco A., Lempp F.A., Tortorici M.A., Pinto D., Walls A.C., Beltramello M., Chen A., Liu Z., Zatta F., Zepeda S., di Iulio J., Bowen J.E., Montiel-Ruiz M., Zhou J., Rosen L.E., Bianchi S., Guarino B., Fregni C.S., Abdelnabi R., Foo S.C., Rothlauf P.W., Bloyet L.M., Benigni F., Cameroni E., Neyts J., Riva A., Snell G., Telenti A., Whelan S.P.J., Virgin H.W., Corti D., Pizzuto M.S., Veesler D. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell. 2021;184(2332–2347) doi: 10.1016/j.cell.2021.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick K.D., Jacobs J.L., Mellors J.W. The emerging plasticity of SARS-CoV-2. Science. 2021;371:1306–1308. doi: 10.1126/science.abg4493. [DOI] [PubMed] [Google Scholar]
- Mercurio I., Tragni V., Busto F., De Grassi A., Pierri C.L. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: from conformational changes to novel neutralizing antibodies. Cell. Mol. Life Sci. 2021;78:1501–1522. doi: 10.1007/s00018-020-03580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad A., Alshawaf E., Marafie S.K., Abu-Farha M., Abubaker J., Al-Mulla F. Higher binding affinity of furin for SARS-CoV-2 spike (S) protein D614G mutant could be associated with higher SARS-CoV-2 infectivity. Int. J. Infect. Dis. 2021;103:611–616. doi: 10.1016/j.ijid.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto F., Sixt T., Devilliers H., Abdallahoui M., Eberl I., Rogier T., Buisson M., Chavanet P., Duong M., Esteve C., Mahy S., Salmon-Rousseau A., Catherine F., Blot M., Piroth L. Is there a need to widely prescribe antibiotics in patients hospitalized with COVID-19? Int. J. Infect. Dis. 2021;105:256–260. doi: 10.1016/j.ijid.2021.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X., Qian L., Sun R., Huang B., Dong X., Xiao Q., Zhang Q., Lu T., Yue L., Chen S., Li X., Sun Y., Li L., Xu L., Li Y., Yang M., Xue Z., Liang S., Ding X., Yuan C., Peng L., Liu W., Yi X., Lyu M., Xiao G., Xu X., Ge W., He J., Fan J., Wu J., Luo M., Chang X., Pan H., Cai X., Zhou J., Yu J., Gao H., Xie M., Wang S., Ruan G., Chen H., Su H., Mei H., Luo D., Zhao D., Xu F., Li Y., Zhu Y., Xia J., Hu Y., Guo T. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184(775–791) doi: 10.1016/j.cell.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J.Y., Jeong H.W., Shin E.C. SARS-CoV-2 mutations, vaccines, and immunity: implication of variants of concern. Signal Transduct. Target Ther. 2021;6:203. doi: 10.1038/s41392-021-00623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink B.B., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., van der Spek A., Tolsma P., Rietveld A., Brouwer M., Bouwmeester-Vincken N., Harders F., Hakze-van der Honing R., Wegdam-Blans M.C.A., Bouwstra R.J., GeurtsvanKessel C., van der Eijk A.A., Velkers F.C., Smit L.A.M., Stegeman A., van der Poel W.H.M., Koopmans M.P.G. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021;371:172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozono S., Zhang Y., Ode H., Sano K., Tan T.S., Imai K., Miyoshi K., Kishigami S., Ueno T., Iwatani Y., Suzuki T., Tokunaga K. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat. Commun. 2021;12:848. doi: 10.1038/s41467-021-21118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z., Ouyang T., Pang D., Ma T., Chen X., Guo N., Chen F., Yuan L., Ouyang H., Ren L. Pseudorabies virus can escape from CRISPR-Cas9-mediated inhibition. Virus Res. 2016;223:197–205. doi: 10.1016/j.virusres.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Pierri C.L. SARS-CoV-2 spike protein: flexibility as a new target for fighting infection. Signal Transduct. Target Ther. 2020;5:254. doi: 10.1038/s41392-020-00369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch J.W., Capoferri A.A., Katusiime M.G., Patro S.C., Kearney M.F. Low genetic diversity may be an Achilles heel of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:24614–24616. doi: 10.1073/pnas.2017726117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa A., Pye V.E., Graham C., Muir L., Seow J., Ng K.W., Cook N.J., Rees-Spear C., Parker E., Dos Santos M.S., Rosadas C., Susana A., Rhys H., Nans A., Masino L., Roustan C., Christodoulou E., Ulferts R., Wrobel A., Short C.E., Fertleman M., Sanders R.W., Heaney J., Spyer M., Kjaer S., Riddell A., Malim M.H., Beale R., MacRae J.I., Taylor G.P., Nastouli E., van Gils M.J., Rosenthal P.B., Pizzato M., McClure M.O., Tedder R.S., Kassiotis G., McCoy L.E., Doores K.J., Cherepanov P. SARS-CoV-2 recruits a haem metabolite to evade antibody immunity. Sci Adv. 2021;7(22) doi: 10.1126/sciadv.abg7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarker S., Nampoothiri M. Structural proteins in severe acute respiratory syndrome Coronavirus-2. Arch. Med. Res. 2020;51:482–491. doi: 10.1016/j.arcmed.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlottau K., Rissmann M., Graaf A., Schon J., Sehl J., Wylezich C., Hoper D., Mettenleiter T.C., Balkema-Buschmann A., Harder T., Grund C., Hoffmann D., Breithaupt A., Beer M. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe. 2020;1:e218–e225. doi: 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segales J., Puig M., Rodon J., Avila-Nieto C., Carrillo J., Cantero G., Terron M.T., Cruz S., Parera M., Noguera-Julian M., Izquierdo-Useros N., Guallar V., Vidal E., Valencia A., Blanco I., Blanco J., Clotet B., Vergara-Alert J. Detection of SARS-CoV-2 in a cat owned by a COVID-19-affected patient in Spain. Proc. Natl. Acad. Sci. U. S. A. 2020;117:24790–24793. doi: 10.1073/pnas.2010817117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyran M., Takayama K., Uversky V.N., Lundstrom K., Palu G., Sherchan S.P., Attrish D., Rezaei N., Aljabali A.A.A., Ghosh S., Pizzol D., Chauhan G., Adadi P., Mohamed Abd El-Aziz T., Soares A.G., Kandimalla R., Tambuwala M., Hassan S.S., Azad G.K., Pal Choudhury P., Baetas-da-Cruz W., Serrano-Aroca A., Brufsky A.M., Uhal B.D. The structural basis of accelerated host cell entry by SARS-CoV-2dagger. FEBS J. 2020 doi: 10.1111/febs.15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Bansal V., Feschotte C. A single-cell RNA expression map of human coronavirus entry factors. Cell Rep. 2020;32:108175. doi: 10.1016/j.celrep.2020.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza P.F.N., Mesquita F.P., Amaral J.L., Landim P.G.C., Lima K.R.P., Costa M.B., Farias I.R., Lima L.B., Montenegro R.C. The human pandemic coronaviruses on the show: the spike glycoprotein as the main actor in the coronaviruses play. Int. J. Biol. Macromol. 2021;179:1–19. doi: 10.1016/j.ijbiomac.2021.02.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.N., Greaney A.J., Addetia A., Hannon W.W., Choudhary M.C., Dingens A.S., Li J.Z., Bloom J.D. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Science. 2021;371:850–854. doi: 10.1126/science.abf9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., He W.T., Wang L., Lai A., Ji X., Zhai X., Li G., Suchard M.A., Tian J., Zhou J., Veit M., Su S. COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol. Med. 2020;26:483–495. doi: 10.1016/j.molmed.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supasa P., Zhou D., Dejnirattisai W., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Nutalai R., Tuekprakhon A., Wang B., Paesen G.C., Slon-Campos J., Lopez-Camacho C., Hallis B., Coombes N., Bewley K.R., Charlton S., Walter T.S., Barnes E., Dunachie S.J., Skelly D., Lumley S.F., Baker N., Shaik I., Humphries H.E., Godwin K., Gent N., Sienkiewicz A., Dold C., Levin R., Dong T., Pollard A.J., Knight J.C., Klenerman P., Crook D., Lambe T., Clutterbuck E., Bibi S., Flaxman A., Bittaye M., Belij-Rammerstorfer S., Gilbert S., Hall D.R., Williams M.A., Paterson N.G., James W., Carroll M.W., Fry E.E., Mongkolsapaya J., Ren J., Stuart D.I., Screaton G.R. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184 doi: 10.1016/j.cell.2021.02.033. 2201–2211 e2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A., Khan A., Gosai F., Asif A., Dhillon S. Gastrointestinal pathophysiology of SARS-CoV2 - a literature review. J. Commun. Hosp. Intern. Med. Perspect. 2020;10:523–528. doi: 10.1080/20009666.2020.1811556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., Wilkinson E., Lessells R.J., Giandhari J., Pillay S., Msomi N., Mlisana K., Bhiman J.N., von Gottberg A., Walaza S., Fonseca V., Allam M., Ismail A., Glass A.J., Engelbrecht S., Van Zyl G., Preiser W., Williamson C., Petruccione F., Sigal A., Gazy I., Hardie D., Hsiao N.Y., Martin D., York D., Goedhals D., San E.J., Giovanetti M., Lourenco J., Alcantara L.C.J., de Oliveira T. Sixteen novel lineages of SARS-CoV-2 in South Africa. Nat. Med. 2021;27:440–446. doi: 10.1038/s41591-021-01255-3. [DOI] [PubMed] [Google Scholar]
- Teruel N., Mailhot O., Najmanovich R.J. Modelling conformational state dynamics and its role on infection for SARS-CoV-2 spike protein variants. bioRxiv. 2021 doi: 10.1101/2020.12.16.423118. 2020.2012.2016.423118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson E.C., Rosen L.E., Shepherd J.G., Spreafico R., da Silva Filipe A., Wojcechowskyj J.A., Davis C., Piccoli L., Pascall D.J., Dillen J., Lytras S., Czudnochowski N., Shah R., Meury M., Jesudason N., De Marco A., Li K., Bassi J., O’Toole A., Pinto D., Colquhoun R.M., Culap K., Jackson B., Zatta F., Rambaut A., Jaconi S., Sreenu V.B., Nix J., Zhang I., Jarrett R.F., Glass W.G., Beltramello M., Nomikou K., Pizzuto M., Tong L., Cameroni E., Croll T.I., Johnson N., Di Iulio J., Wickenhagen A., Ceschi A., Harbison A.M., Mair D., Ferrari P., Smollett K., Sallusto F., Carmichael S., Garzoni C., Nichols J., Galli M., Hughes J., Riva A., Ho A., Schiuma M., Semple M.G., Openshaw P.J.M., Fadda E., Baillie J.K., Chodera J.D., Investigators I.C., Consortium C.-G.U., Rihn S.J., Lycett S.J., Virgin H.W., Telenti A., Corti D., Robertson D.L., Snell G. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity. Cell. 2021;184(1171–1187) doi: 10.1016/j.cell.2021.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turonova B., Sikora M., Schurmann C., Hagen W.J.H., Welsch S., Blanc F.E.C., von Bulow S., Gecht M., Bagola K., Horner C., van Zandbergen G., Landry J., de Azevedo N.T.D., Mosalaganti S., Schwarz A., Covino R., Muhlebach M.D., Hummer G., Krijnse Locker J., Beck M. In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges. Science. 2020;370:203–208. doi: 10.1126/science.abd5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl. Res. 2020;226:57–69. doi: 10.1016/j.trsl.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena J., Li C., Vizoso-Pinto M.G., Sacur J., Ren L., Kitazawa H. Lactiplantibacillus plantarum as a potential adjuvant and delivery system for the development of SARS-CoV-2 Oral vaccines. Microorganisms. 2021;9:683. doi: 10.3390/microorganisms9040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(281–292) doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Fu T., Hao J., Li L., Tian M., Jin N., Ren L., Li C. A recombinant lactobacillus plantarum strain expressing the spike protein of SARS-CoV-2. Int. J. Biol. Macromol. 2020;160:736–740. doi: 10.1016/j.ijbiomac.2020.05.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.Y., Zhao R., Gao L.J., Gao X.F., Wang D.P., Cao J.M. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front. Cell. Infect. Microbiol. 2020;10:587269. doi: 10.3389/fcimb.2020.587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Qiu Y., Li J.Y., Zhou Z.J., Liao C.H., Ge X.Y. A unique protease cleavage site predicted in the spike protein of the novel pneumonia coronavirus (2019-nCoV) potentially related to viral transmissibility. Virol. Sin. 2020;35:337–339. doi: 10.1007/s12250-020-00212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Hozumi Y., Zheng Y.H., Yin C., Wei G.W. Host immune response driving SARS-CoV-2 evolution. Viruses. 2020;12:1095. doi: 10.3390/v12101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Trilling M., Sutter K., Dittmer U., Lu M., Zheng X., Yang D., Liu J. A crowned Killer’s resume: genome, structure, receptors, and origin of SARS-CoV-2. Virol. Sin. 2020;35:673–684. doi: 10.1007/s12250-020-00298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., Graham B.S., Mascola J.R., Chang J.Y., Yin M.T., Sobieszczyk M., Kyratsous C.A., Shapiro L., Sheng Z., Huang Y., Ho D.D. Increased resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7 to antibody neutralization. Nature. 2021;593(7857):130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- Wang S., Qiu Z., Hou Y., Deng X., Xu W., Zheng T., Wu P., Xie S., Bian W., Zhang C., Sun Z., Liu K., Shan C., Lin A., Jiang S., Xie Y., Zhou Q., Lu L., Huang J., Li X. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021;31:126–140. doi: 10.1038/s41422-020-00460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Viant C., Gaebler C., Cipolla M., Hoffmann H.H., Oliveira T.Y., Oren D.A., Ramos V., Nogueira L., Michailidis E., Robbiani D.F., Gazumyan A., Rice C.M., Hatziioannou T., Bieniasz P.D., Caskey M., Nussenzweig M.C. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D., Alameh M.G., de Silva T., Collini P., Hornsby H., Brown R., LaBranche C.C., Edwards R.J., Sutherland L., Santra S., Mansouri K., Gobeil S., McDanal C., Pardi N., Hengartner N., Lin P.J.C., Tam Y., Shaw P.A., Lewis M.G., Boesler C., Sahin U., Acharya P., Haynes B.F., Korber B., Montefiori D.C. D614G spike mutation increases SARS CoV-2 susceptibility to neutralization. Cell Host Microbe. 2021;29(23–31) doi: 10.1016/j.chom.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibmer C.K., Ayres F., Hermanus T., Madzivhandila M., Kgagudi P., Oosthuysen B., Lambson B.E., de Oliveira T., Vermeulen M., van der Berg K., Rossouw T., Boswell M., Ueckermann V., Meiring S., von Gottberg A., Cohen C., Morris L., Bhiman J.N., Moore P.L. SARS-CoV-2 501Y.V2 escapes neutralization by south African COVID-19 donor plasma. Nat Med. 2021;27(4):622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- Wu L., Chen Q., Liu K., Wang J., Han P., Zhang Y., Hu Y., Meng Y., Pan X., Qiao C., Tian S., Du P., Song H., Shi W., Qi J., Wang H.W., Yan J., Gao G.F., Wang Q. Broad host range of SARS-CoV-2 and the molecular basis for SARS-CoV-2 binding to cat ACE2. Cell Discov. 2020;6:68. doi: 10.1038/s41421-020-00210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Liu Y., Liu J., Zhang X., Zou J., Fontes-Garfias C.R., Xia H., Swanson K.A., Cutler M., Cooper D., Menachery V.D., Weaver S.C., Dormitzer P.R., Shi P.Y. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat. Med. 2021;27:620–621. doi: 10.1038/s41591-021-01270-4. [DOI] [PubMed] [Google Scholar]
- Xu P., Liu X., Zhang X., Ji W., Ren L. Research Progress on SARS-CoV-2. China Ani. Health Insp. 2020;37:67–72. [Google Scholar]
- Yeoh Y.K., Zuo T., Lui G.C., Zhang F., Liu Q., Li A.Y., Chung A.C., Cheung C.P., Tso E.Y., Fung K.S., Chan V., Ling L., Joynt G., Hui D.S., Chow K.M., Ng S.S.S., Li T.C., Ng R.W., Yip T.C., Wong G.L., Chan F.K., Wong C.K., Chan P.K., Ng S.C. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovetskiy L., Wang X., Pascal K.E., Tomkins-Tinch C., Nyalile T.P., Wang Y., Baum A., Diehl W.E., Dauphin A., Carbone C., Veinotte K., Egri S.B., Schaffner S.F., Lemieux J.E., Munro J.B., Rafique A., Barve A., Sabeti P.C., Kyratsous C.A., Dudkina N.V., Shen K., Luban J. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Cell. 2020;183(739–751) doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X., Sun J., Yan Z., Zhang J., Zhao J., Zhao Z., Gao Q., He W.T., Veit M., Su S. Comparison of severe acute respiratory syndrome coronavirus 2 spike protein binding to ACE2 receptors from human, pets, farm animals, and putative intermediate hosts. J. Virol. 2020;94 doi: 10.1128/JVI.00831-20. e00831–00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Jackson C.B., Mou H., Ojha A., Peng H., Quinlan B.D., Rangarajan E.S., Pan A., Vanderheiden A., Suthar M.S., Li W., Izard T., Rader C., Farzan M., Choe H. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat. Commun. 2020;11:6013. doi: 10.1038/s41467-020-19808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Qiao S., Yu J., Zeng J., Shan S., Tian L., Lan J., Zhang L., Wang X. Bat and pangolin coronavirus spike glycoprotein structures provide insights into SARS-CoV-2 evolution. Nat. Commun. 2021;12:1607. doi: 10.1038/s41467-021-21767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Shi Z.L. SARS-CoV-2 spillover events. Science. 2021;371:120–122. doi: 10.1126/science.abf6097. [DOI] [PubMed] [Google Scholar]
- Zhou B., Thao T.T.N., Hoffmann D., Taddeo A., Ebert N., Labroussaa F., Pohlmann A., King J., Steiner S., Kelly J.N., Portmann J., Halwe N.J., Ulrich L., Trueb B.S., Fan X., Hoffmann B., Wang L., Thomann L., Lin X., Stalder H., Pozzi B., de Brot S., Jiang N., Cui D., Hossain J., Wilson M.M., Keller M.W., Stark T.J., Barnes J.R., Dijkman R., Jores J., Benarafa C., Wentworth D.E., Thiel V., Beer M. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature. 2021;592:122–127. doi: 10.1038/s41586-021-03361-1. [DOI] [PubMed] [Google Scholar]
- Zhou D., Dejnirattisai W., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Tuekprakhon A., Nutalai R., Wang B., Paesen G.C., Lopez-Camacho C., Slon-Campos J., Hallis B., Coombes N., Bewley K., Charlton S., Walter T.S., Skelly D., Lumley S.F., Dold C., Levin R., Dong T., Pollard A.J., Knight J.C., Crook D., Lambe T., Clutterbuck E., Bibi S., Flaxman A., Bittaye M., Belij-Rammerstorfer S., Gilbert S., James W., Carroll M.W., Klenerman P., Barnes E., Dunachie S.J., Fry E.E., Mongkolsapaya J., Ren J., Stuart D.I., Screaton G.R. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184 doi: 10.1016/j.cell.2021.02.037. 2348–2361 e2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.