Fig 4. Low-dose LGG pre-treatment induces SIGIRR and ameliorates TLR-mediated inflammation in mice with experimental NEC.
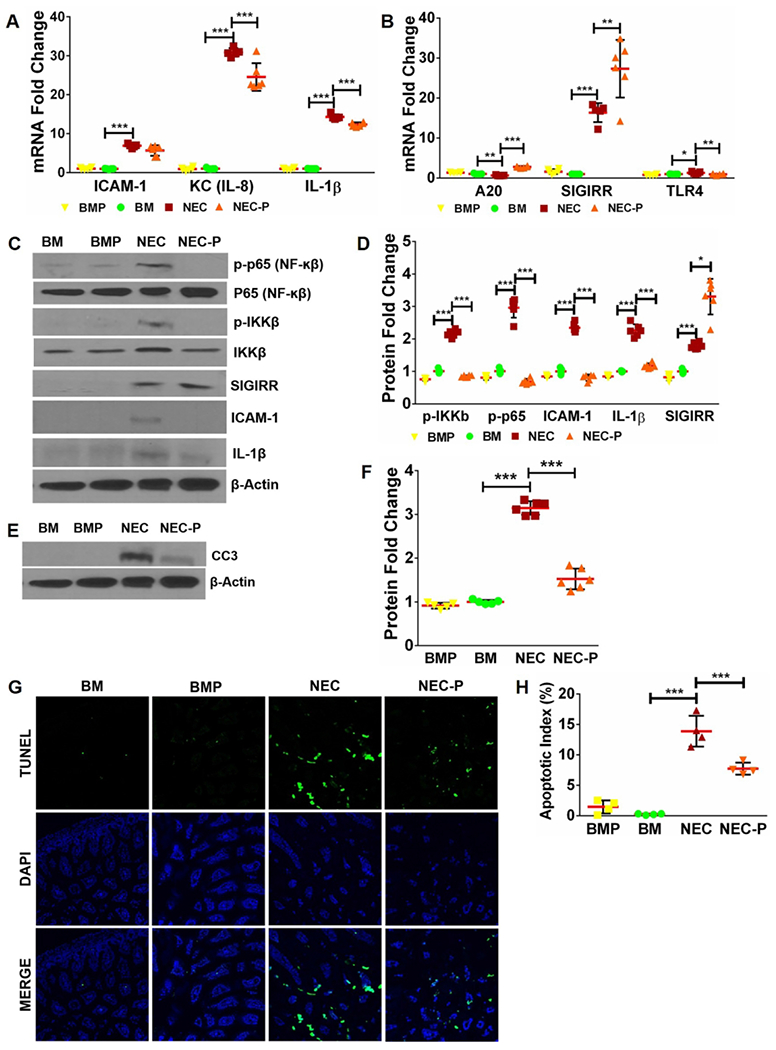
(A) Gene expression of inflammatory cytokines ICAM1, KC, and IL-1β increased with NEC and decreased in NEC-P group pre-treated with LGG. (n≥5 per group; ***p<0.001). (B) Pups pre-treated with LGG exhibit higher SIGIRR and A20 expression compared to untreated mice with NEC. (n≥5 per group. ***p<0.001, **p<0.01, *p<0.05). (C) Western blot analysis of canonical TLR4 signaling mediators and downstream inflammatory effectors demonstrated increased levels of phosphorylated p65, phosphorylated IKKβ, ICAM1, and IL-1β with NEC induction, all of which were attenuated in mice pre-treated with LGG. Increased SIGIRR protein expression in mice pre-treated with LGG (NEC-P) was also evident. (D) Graphical representation of densitometric quantification of protein expression showing decreased canonical TLR4 inflammatory signaling in mice pre-treated with LGG prior to NEC induction. (n≥5 per group. ***p<0.001, *p<0.05). (E) CC3 protein expression of BM and NEC mice at baseline and following LGG treatment was examined by western blotting of ileal homogenates. (F) Quantification of CC3 protein using densitometry demonstrated increased CC3 levels with NEC that were attenuated with LGG treatment. (n≥5 per group. ***p<0.001). (G) Representative ileal sections from BM, BMP, NEC, and NEC-P pups show reduction in apoptotic cells (TUNEL, green stained) with LGG pre-treatment. (H) Quantification of apoptotic index (apoptotic cells/total cells) in intestinal sections demonstrate statistically significant decrease in apoptotic index in NEC-P mice compared to NEC. (n≥4 per group; ***p<0.001, **p<0.01).
