Figure 1.
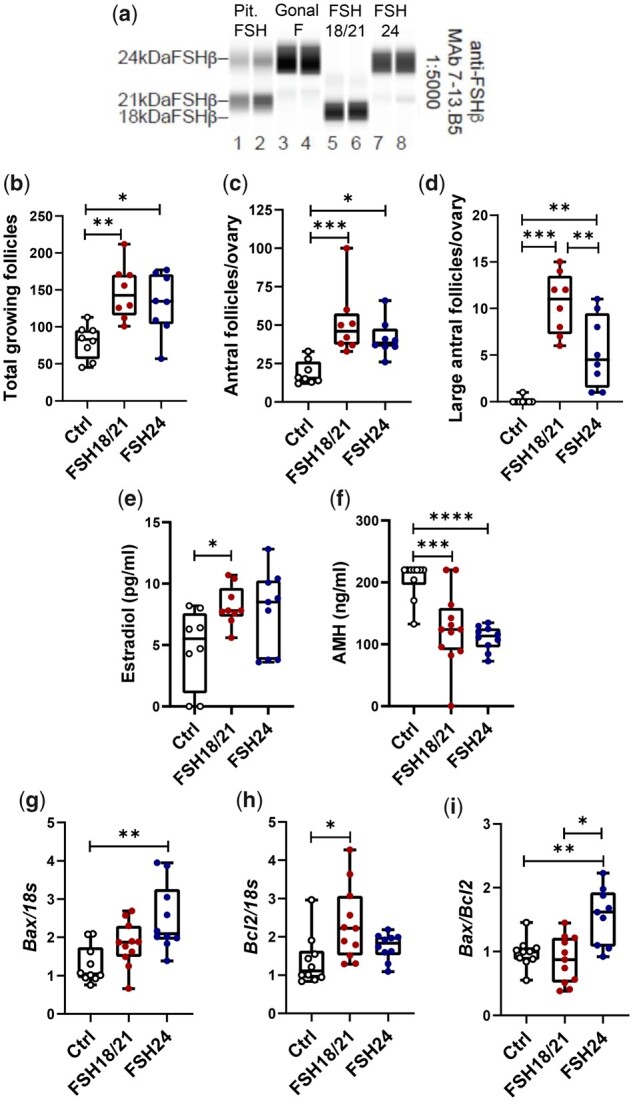
The effects of hypo- and fully-glycosylated human recombinant FSH (hFSH) on follicular development in vivo. (a) Automated western blot of recombinant hFSH preparations. The primary FSHβ antibody was 7-13.B5 diluted 1:5000. Lanes 1 and 2, 100 ng pituitary FSH AFP7298A; lanes 3 and 4, 100 ng Gonal-f® (FSH used in clinical ART); lanes 5 and 6, 100 ng hFSH18/21; lanes 7 and 8, 100 ng hFSH24. (b) Total growing follicles, including pre-antral follicles, and antral follicles, 48 h after treatment with hFSH18/21 and hFSH24. (c) Numbers of antral follicles per ovary in control, hFSH18/21 and hFSH24 treatment groups. (d) Number of large antral follicles per ovary in control, hFSH18/21 and hFSH24 treatment groups. (e) Serum estradiol concentrations (pg/ml) in different treatment groups. (f) Serum anti-Müllerian hormone (AMH) concentrations (ng/ml) in different treatment groups. (g–i) Total RNA was extracted for qRT-PCR in Control, hFSH18/21, and hFSH24 treatment groups. (g) Ovarian transcripts for Bax. (h) Ovarian transcripts for Bcl2. (i) Bax versus Bcl2 gene expression ratio. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
