Abstract
Background
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are life-threatening diseases and could occur in severe COVID-19 patients. Re-Du-Ning injection (RDN) is a tradition Chinese medicine preparation which has been clinically used for treatment of respiratory diseases including COVID-19.
Purpose
To elucidate the potential mechanisms of RDN for the treatment of ALI.
Methods
Female C57BL/6J mice were used to establish ALI model by intraperitoneal injection 10 mg/kg LPS, and RDN injection was intraperitoneally administered with the dose of 5 and 10 ml/kg. The cytokines were measured by ELISA and qPCR. The data related to NETs were analyzed by ELISA, immunofluorescence, Western blotting and network pharmacological approach.
Results
RDN robustly alleviated LPS-induced ALI. Meanwhile, RDN downregulated the expression of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α. Specifically, RDN treatment inhibited the formation of neutrophil extracellular traps (NETs) and remarkably suppressed the protein of PAD4. The active compound from RDN decreased the phosphorylation of ERK1/2.
Conclusion
These findings demonstrate that RDN ameliorates LPS-induced ALI through suppressing MAPK pathway to inhibit the formation of NETs.
Keywords: Re-Du-Ning injection, ALI, ARDS, NETs, MAPK
Abbreviations: ALI, Acute lung injury; ARDS, Acute respiratory distress syndrome; BALF, bronchoalveolar lavage fluid; cit H3, Citrullinated histone H3; COVID-19, Coronavirus Disease 2019; Dex, Dexamethasone; ELISA, Enzyme-linked immunosorbent assay; ERK1/2, Extracellular signal regulated kinases; GM-CSF, Granulocyte-macrophage Colony Stimulating Factor; H&E., Hematoxylin-eosin; i.p, Intraperitoneal injection; IL-1β, Interleukin (IL)-1β; IL-6, Interleukin (IL)-6; IL-17A, Interleukin (IL)-17A; LPS, Lipopolysaccharide; MAPK, Mitogen-activated protein kinase; MPO, Myeloperoxidase; NE, neutrophil elastase; NETs, neutrophil extracellular traps; PAD4, peptidyl arginine deiminase 4; PMA, phorbol 12-myristate 13-acetate; RDN, Re-Du-Ning injection; TNF-α, Tumor necrosis factor α
Graphical abstract
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are still the main cause of mortality in patients with severe lung diseases in the world (Liu et al., 2018; Matthay et al., 2012). Inflammation is one of the main reasons for ALI (Mikacenic et al., 2018). ALI can cause a large number of neutrophils flooding into the lung, releasing pro-inflammatory cytokines, damaging the lung epithelium and endothelium, increasing gas exchange and leading to pulmonary edema, which is an excessive inflammatory response (Gong et al., 2014; Jin et al., 2007). ARDS often occurs in cases of pneumonia, sepsis, aspiration of gastric contents or severe trauma (Matthay et al., 2019).
Neutrophils are the first barrier against inflammation. They include three main functions: phagocytosis, degranulation and release of neutrophil extracellular traps (NETs). NETs are reticular, extracellular structures consist of cytosolic and granule proteins Papayannopoulos (2018). In mouse model of acute lung injury, a large number of NETs were detected in the lung tissue. (Mikacenic et al., 2018; Liu et al., 2016). In 2019, the coronavirus disease (COVID-19) is spreading all over the world, and the death rate of patients remains high. Notably, the main cause of death is that patients infected with COVID are transformed into acute respiratory distress syndrome in the later stage, and the normal function of lung tissue is lost. Recent evidence suggests that the formation of NETs in COVID-19 patients has increased (Veras et al., 2020).
Re-Du-Ning injection (RDN) is a traditional Chinese medicine preparation, consisting of Lonicera Japonica Thunb., Gardenia Jasminoides Ellis and Artemisia Annua L. (Gao et al., 2019; Geng et al., 2015). Artemisia Annua L. can promote cellular immunity. Lonicera Japonica Thunb. has the effects on clearing heat, detoxicating and dispelling cold. Gardenia Jasminoides Ellis has the effects on protecting liver, hemostasis and detumescence. RDN is clinically used to treat cough, cold and upper respiratory diseases (Gao et al., 2019). It has the effects on clearing heat and detoxicating. Previous research indicates RDN moderates paraquat-induced acute lung injury through inhibition of the AMPK/MAPK/NF-κB signaling pathway (Jiang et al., 2019). However, the ameliorative effect and mechanism of RDN on ALI are still unclear. In the present study, we demonstrated that RDN alleviated LPS-induced ALI through suppressing MAPK pathway to inhibit the formation of NETs.
Methods and materials
Animals
Eight-week-old female C57BL/6J mice were purchased from GemPharmatech Co. Ltd. (Nanjing, China). Mice were fed water and standard chow in a specific, pathogen-free room. A total of forty mice were used in this study and the mice were acclimatized to the standard laboratory conditions for one week prior to experiments.
Agents
Re-Du-Ning injection and some related-compounds were provided by Kanion Pharmaceutical Co., Ltd. (Lianyungang, China). LPS (from Escherichia coli (0111:B4)) and Dexamethasone were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cl-amidine and GSK484 were bought from Selleck Chemicals (Houston, TX, USA). ELISA kits for mouse IL-1β, IL-6 and TNF-α were purchased from Dakewei (Beijing, China); Human MPO ELISA kit was bought from Lianke (Hangzhou, China); Mouse NE and MPO kits were purchased from R&D Systems (Minneapolis, MN, USA). Anti-rabbit phosphorylation of ERK1/2 (Thr202/Tyr204) monoclonal antibody and anti-rabbit ERK1/2 monoclonal antibody were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-mouse PAD4 monoclonal antibody, anti-rabbit Histone H3 polyclonal antibody and anti-mouse Myeloperoxidase (MPO) monoclonal antibody was purchased from Abcam (Cambridge, UK). Alexa Fluor 488-conjugated goat anti-rabbit IgG (H+L) cross-absorbed secondary Ab and Alexa Fluor 647-conjugated goat anti-mouse IgG (H+L) cross-absorbed secondary Ab were purchased from Fumaisi (Nanjing, China). Anti-mouse β-actin monoclonal antibody and anti-mouse GAPDH monoclonal antibody were purchased from Abmart (Shanghai, China). Anti-mouse CD3-AF488, F4/80-APC, Ly6G-PE/Cy7 antibodies were purchased from Biolegend (San Diego, CA, USA). Anti-mouse CD45-AF700 and CD11b-PE antibodies were purchased from BD Pharmingen (San Diego, CA, USA).
High performance liquid chromatography (HPLC) analysis of RDN
Chlorogenic acid (10753-201716, 99.3%), geniposide (110749-201617, 97.6%), geniposidic acid (111828-201604, 97.4%), caffeic acid (110885-201703, 99.7%), isochlorogenic acid A (111782-201706, 97.3%) and isochlorogenic acid C (111894-2010012, 89.6%) were purchased from National Institutes for Food and Drug Control (Beijing, China). Neochlorogenic acid (P13O8F45676, 99.6%), cryptochlorogenic acid (Y12O8H45678, 98.5%), shanzhiside (P30J9F64637, 99.4%) and isochlorogenic acid B (P20J9F53191, 97.3%) were purchased from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). Secoxyloganin (17033103, 98.5%) and genipin-1-O-β-D-gentiobioside (18051408, 98.1%) were purchased from Chengdu Pufei De Biotech Co., Ltd (Chengdu, China). Secologanic acid (5685, 96.78%) was purchased from Shanghai shidande Standard Technology Service Co., Ltd (Shanghai, China).
The fingerprint of Re-Du-Ning injection was established by using sample chromatography of 10 different production batches. HPLC analysis was performed on a Dionex UltiMate 3000 high performance liquid chromatography (Thermo Fisher Scientific, San Jose, CA, USA) comprising a quaternary pump, a diode-array detector (DAD), an autosampler, and a column compartment. Samples were separated on an a Agilent ZORBAX Eclipse Plus-C18) 4.6 × 250 mm, 5 μm, Agilent, USA(;The mobile phase consisted of methanol (A) and water containing 0.1% formic acid (B). A gradient program was used as follows: 0–10 min, 15–25% A; 10–60 min, 25–50% A; 60–65 min, 50–100% A; 65–70 min, 100–100% A; 70–75 min, 100–15% A. The flow rate was 0.8 ml/min. The column temperature was 30 ℃. The DAD detector scanned from 190 to 400 nm, and the samples were detected at 220 nm.
Mouse acute lung injury model
For LPS-induced lung injury, mice were injected intraperitoneally (i.p.) with 100 μl of 10 mg/kg LPS. In addition, the enunciative concentration of RDN injection or vehicle was diluted in 100 μl sterile PBS and injected i.p. into the mice 1 h after the LPS application. Mice were euthanized 24 h after administration of LPS.
Bronchoalveolar lavage fluid analysis by flow cytometry
The lung tissues of mice were perfused with ice PBS and washed three times. The total number of BALF cells was evaluated and the BALF cells were incubated with anti-mouse CD45-AF700, CD3-AF488, CD11b-PE, F4/80-APC and Ly6G-PE/Cy7 in PBS. Fluorescence-activated cell sorting (FACS) analysis was used to detect the number of immune cells.
Histological analysis
The mouse lungs were fixed in 10% formalin and removed for paraffin, and then sections were stained with hematoxylin and eosin. The pathologic scores were based on published criteria (Szapiel et al.,1979)
Cytokine analysis
Serum and BALF samples were measured by instructions of ELISA kits.
Real-time PCR
RNA was extracted from the mouse lung and reversed to cDNA. cDNA was analyzed by qPCR using SYBR Green Realtime PCR Master Mix (Vazyme Biotech Co., Ltd., China) with the BioRad CFX Connect Real-Time System (Bio-Rad, Hercules, CA). The amplification program was 1 cycle of 95 °C for 2.5 min followed by 44 cycles of 95 °C for 15 s and 60 °C for 30 s. The murine primers used for RT-PCR were as follows:
β-actin, 5’-GGCTGTATTCCCCTCCATCG and 3’- CCAGTTGGTAACAATGCCATGT;
Il-1β, 5’- GAAATGCCACCTTTTGACAGTG and 3’- TGGATGCTCTCATCAGGACAG;
Il-6, 5’- CTGCAAGAGACTTCCATCCAG and 3’- AGTGGTATAGACAGGTCTGTTGG;
Tnf-α, 5’- CCTGTAGCCCACGTCGTAG and 3’- GGGAGTAGACAAGGTACAACCC.
Western blot
Protein was isolated from lung tissue in lysis buffer. Samples were tested by BCA protein assay Kit (Pierce, Rochford, IL). Supernatant were run on the SDS-PAGE and transferred onto PVDF membranes. Membrane was blocked with 5% BSA and incubated with primary antibodies overnight at 4 ℃. After washing, membranes were incubated with HRP-coupled secondary antibody for 90 min. LumiGLO chemiluminescent substrate system was used for detection.
Immunofluorescence
Tissue sections were blocked in Blocking buffer (1 × PBS, 5% anti-goat serum, 0.01% Triton X-100) for 1 h, and then incubated with the primary antibodies at 4 ℃ overnight. The sections were washed 3 times with PBST and used the secondary antibodies for 90 min at room temperature. The nuclei were stained with DAPI. Samples were imaged with a confocal microscope (Carl Zeiss, Jena, Germany.)
Human neutrophil isolation
Neutrophils were isolated from the peripheral blood of healthy donors and used immediately. Neutrophils were obtained by instruction of Human Neutrophil Isolation Kit (Haoyang, Tianjin, China).
Network pharmacological approach
The chemical ingredients from RDN were collected from TCMSP (http://tcmspw.com/) and DrugBank (https://drugbank.ca/). All targets related to ALI was collected from Genecards. The protein-protein interaction (PPI) network was visualized by Cytoscape (Version 3.8.0) software. KEGG enrichment analysis was performed on bioinformatics (http://bioinformatics.com.cn/).
Statistical analysis
GraphPad Prism version 7.0 was used for depictive statistical analysis. Data are presented as mean ± SEM and were analyzed by Student's t-test method to evaluate the diversity between 2 groups. P values less than 0.05 were considered significant.
Results
Identification of the components in RDN
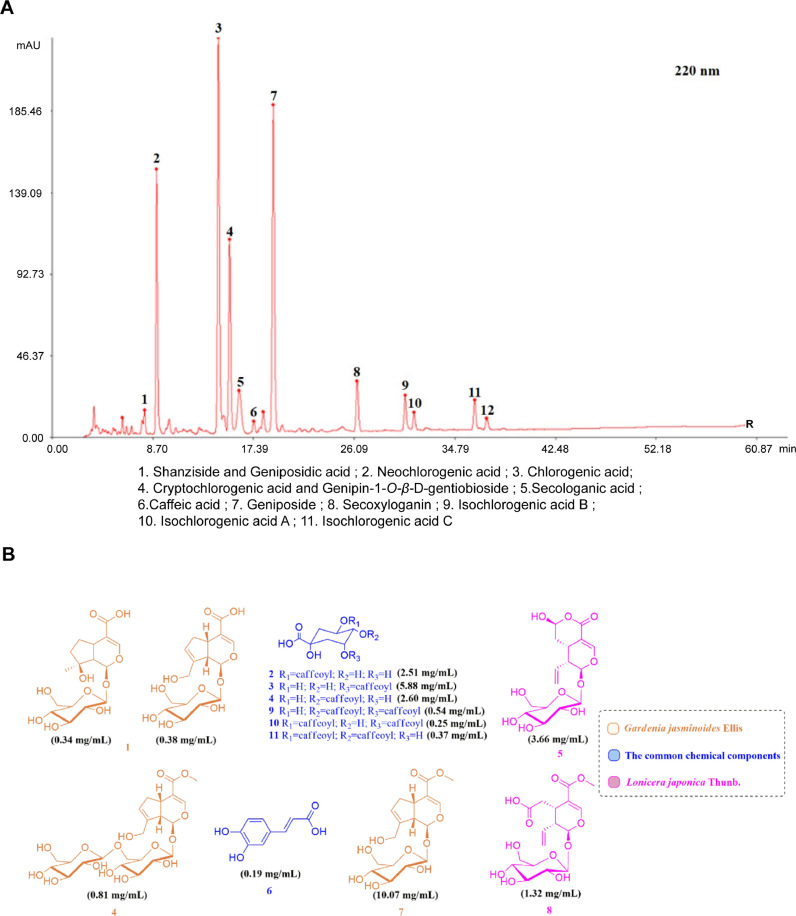
The HPLC analysis of 1 ml Re-Du-Ning showed these constituents: 0.34 mg shanzhiside, 0.38 mg geniposidic acid, 2.51 mg neochlorogenic acid, 5.88 mg chlorogenic acid, 2.60 mg cryptochlorogenic acid, 0.81 mg Genipin-1-O-β-D-gentiobioside, 3.66 mg secologanic acid, 0.19 mg caffeic acid, 10.07 mg geniposide, 1.32 mg secoxyloganin, 0.54 mg isochlorogenic acid B, 0.25 mg isochlorogenic acid A and 0.37 mg isochlorogenic acid C (Fig. 1 A). The major detected structures of the compounds from Re-Du-Ning could be revealed in Fig. 1B.
Fig. 1.
HPLC analysis of the compounds from RDN. (A) HPLC fingerprints of RDN. (B) Chemical structures from RDN.
RDN injection ameliorated LPS-induced lung injury and improved survival in mice
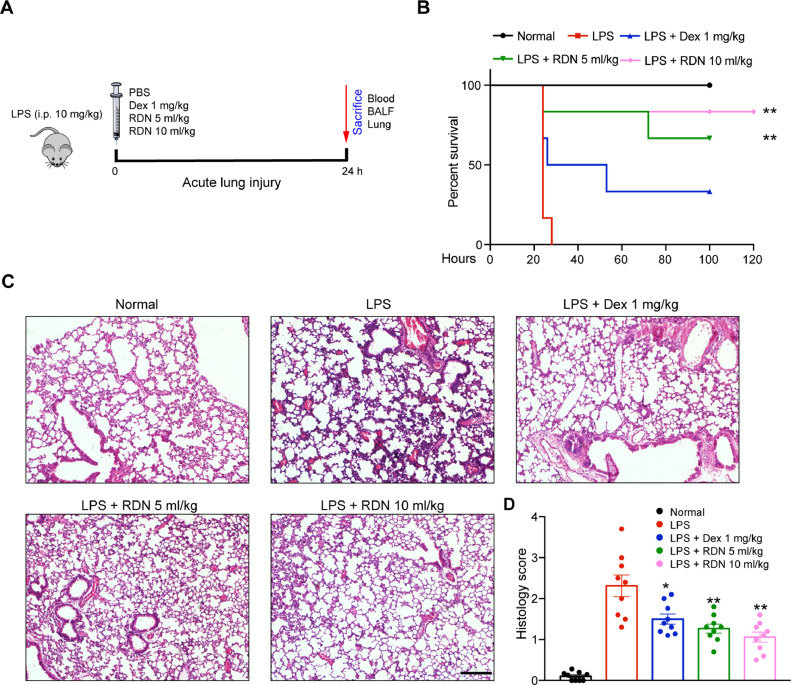
To assess the in vivo impact of RDN injection on lung, we used LPS-induced acute lung injury model (Fig. 2 A). Compared with LPS group, RDN significantly improved survival of mice (Fig. 2B). The lung histological studies also showed that administration of RDN reduced peribronchial and perivascular leukocyte infiltration (Fig. 2C, D). These data suggested that RDN can ameliorate LPS-induced acute lung injury.
Fig. 2.
Administration of RDN improved survival and reduced inflammation score during ALI. (A) Mice were intraperitoneally administered by Reduning injection (5 and 10 ml/kg) and Dex (1 mg/kg) for day 0. The animals were euthanized 24 h after LPS instillation. (B) Kaplan–Meier survival analysis of mice in different groups, n = 8 per group. (C) H&E staining of lung sections from each group, respectively. Scale bar, 100 μm. Values are shown as mean ± SEM of 8 mice. * p < 0.05, ** p < 0.01.
RDN injection inhibited infiltration of inflammatory cells
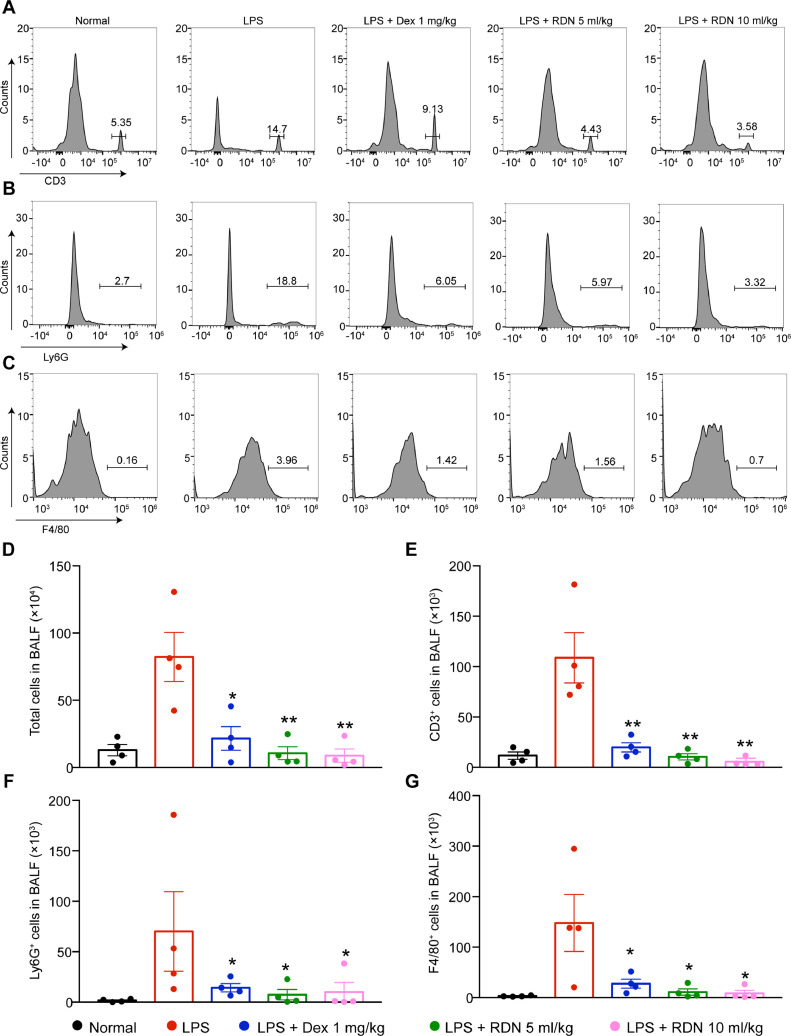
Administration of LPS in C57BL/6 for 24 h can cause severe acute lung inflammation, which leads to an increase in the number of BALF cells (T cells, macrophages and neutrophils). However, RDN led to significant and dose-dependent reductions in T cells (Fig. 3 A, E), neutrophils (Fig. 3B, F), macrophages (Fig. 3C, G) and total cell numbers (Fig. 3D).
Fig. 3.
Administration of RDN suppressed immune cell infiltration in lung. Flow cytometry of CD45+CD3+ (A), CD45+CD11b+Ly6G+ (B) and CD45+CD11b+F4/80+ (C). Statistical analysis of cells in BALF (D), T lymphocytes (E), neutrophils (F) and macrophages (G). Data are shown as the mean ± SEM, n = 4 mice per group. * p < 0.05, ** p < 0.01 vs. the LPS group.
RDN injection ameliorated inflammation in lung
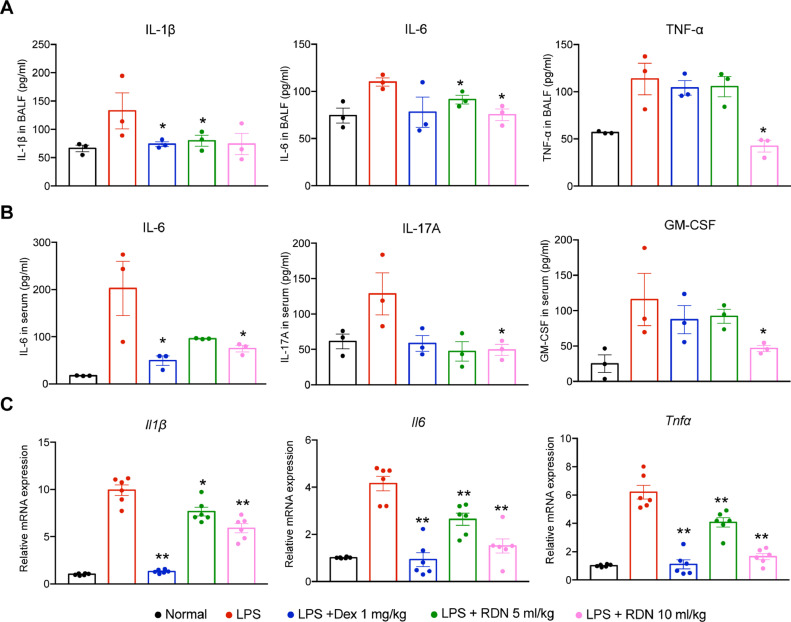
To explore the impacts of inflammatory cytokines, we detected typical cytokines in the plasma and BALF, such as IL-1β, IL-6, TNF-α, IL-17A and GM-CSF. The LPS group showed significantly high levels of inflammatory cytokines in the serum and BALF. However, administration of RDN effectively reduced the levels of cytokines contrasted with the LPS group (Fig. 4 A, B). The mRNA expressions of cytokines, such as IL-1β, IL-6 and TNF-α, were remarkably elevated after LPS challenge. RDN decreased these cytokines in a dose-dependent manner (Fig. 4C).
Fig. 4.
RDN reduced LPS-induced cytokine secretion. Cytokine levels in BALF (A) and serum (B) were measured by using ELISA. Values are shown as the mean ± SEM of 3 mice. (C) The cytokine mRNA expression was measured in lung homogenates by using qPCR. Data are expressed as the mean ± SEM of 6 mice. * p < 0.05, ** p < 0.01 vs. the LPS group.
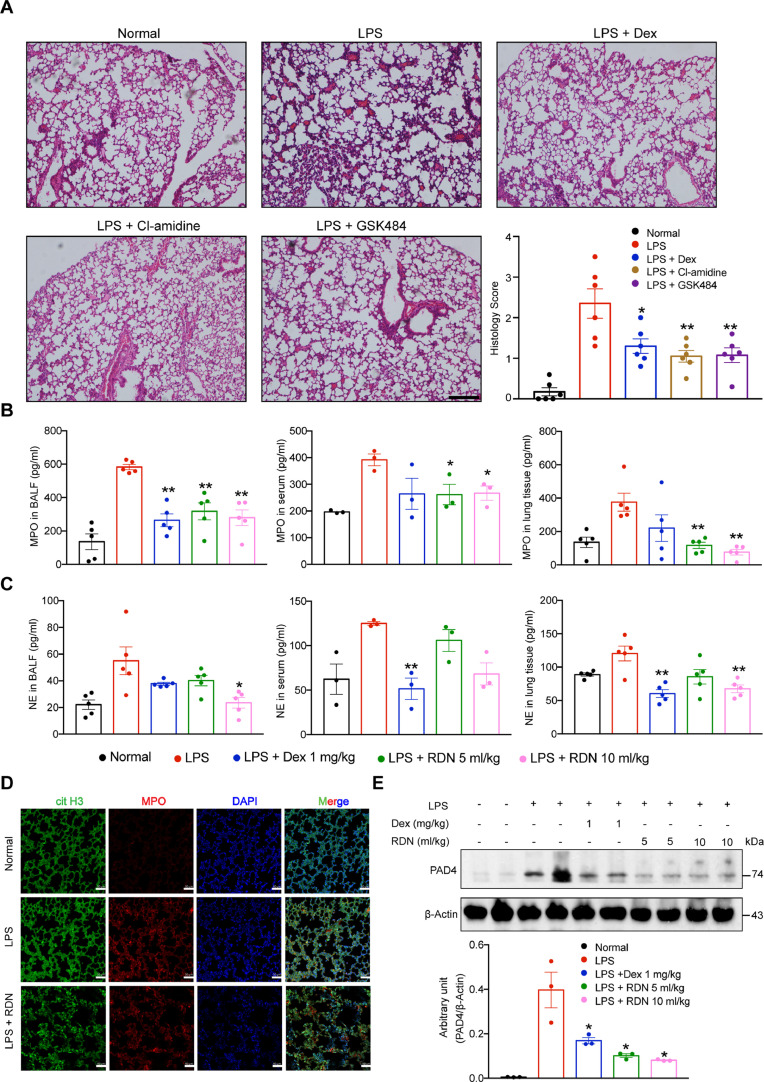
RDN injection reduced release of NETs
NETs formation can occur in LPS-induced lung injury in mouse model (Khan et al., 2017). We inhibited NETosis by treating mice with Cl-amidine and GSK484, both PAD4 inhibitors. The Cl-amidine and the GSK484 group dramatically mitigated inflammatory pathological changes in lung tissues (Fig. 5 A). We measured the levels of NE and MPO in the serum, BALF and lung tissue, and also found they were significantly decreased after RDN treatment (Fig. 5B, C). Next, we performed immunofluorescence staining of lung sections and observed that NETs formation in RDN group was reduced compared with the LPS group (Fig. 5D). Western blot analysis revealed that the protein levels of PAD4 decreased after RDN administration compared to LPS injection in mice (Fig. 5E).
Fig. 5.
RDN reduced the release of neutrophil extracellular traps. (A) Mice were intraperitoneally administered by Cl-amidine (20 mg/kg) and GSK484 (4 mg/kg) for day 0. The animals were euthanized 24 h after LPS instillation. H&E staining of lung sections from each group, respectively. Scale bar, 100 μm. Concentration of MPO (B) and NE (C) in BALF, serum and lung tissue were determined by ELISA. (D) Immunofluorescence staining of lung tissue sections from mice was performed, with imaging for cit H3 (green) and myeloperoxidase (MPO; red) and staining with DAPI for DNA-blue. Scale bar, 50 μm. (E) Immunoblot analysis of the PAD4 protein levels from lung tissues of each group. Data are expressed as the mean ± SEM of 5-6 mice. * p < 0.05, ** p < 0.01 vs. the LPS group.
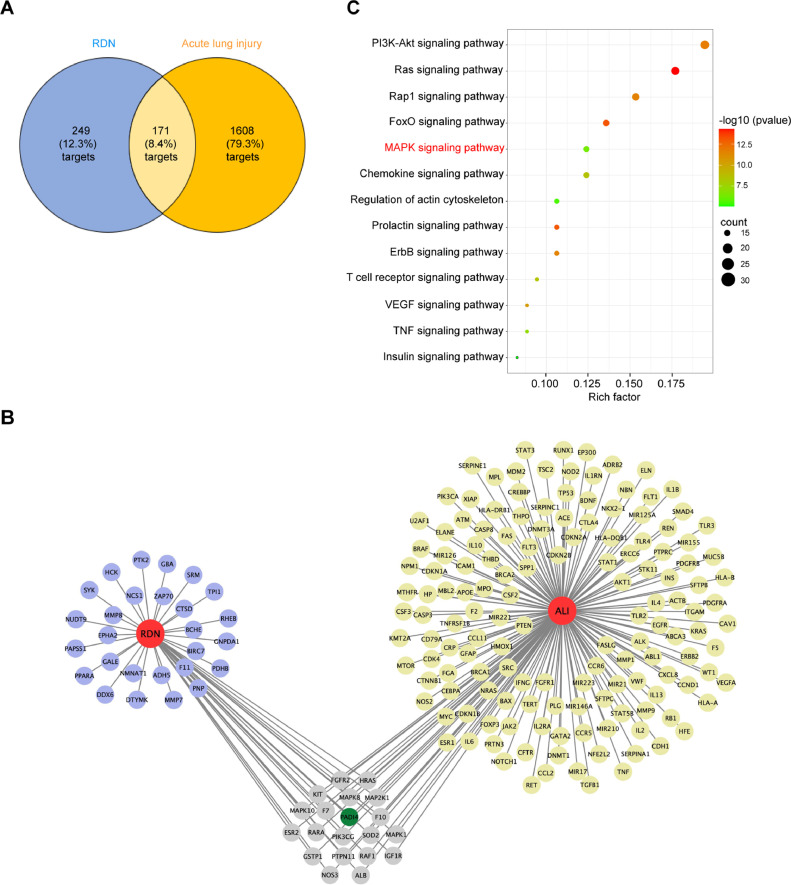
Drug-target, disease-target and target-pathway network analysis
In order to obtain the targets of RDN, we collected from TCMSP and DrugBank database. A total of 249 RDN-related targets were obtained. The targets entries related to acute lung injury were collected from the GeneCards database and 1608 targets were obtained. As a result, there were 171 targets in overlapping part between the targets of RDN and acute lung injury (Fig. 6 A). Some typical targets, such as PADI4 etc, were shown in Fig. 6B. Next, we analyzed 171 mutual targets via KEGG pathway enrichment (Fig. 6C).
Fig. 6.
Network pharmacology analysis of RDN. (A) The number of Reduning and acute lung injury targets. (B) The blue circle represents the RDN-related biomarkers; the yellow circle represents the biomarkers of acute lung injury. (C) KEGG pathway enrichment analysis.
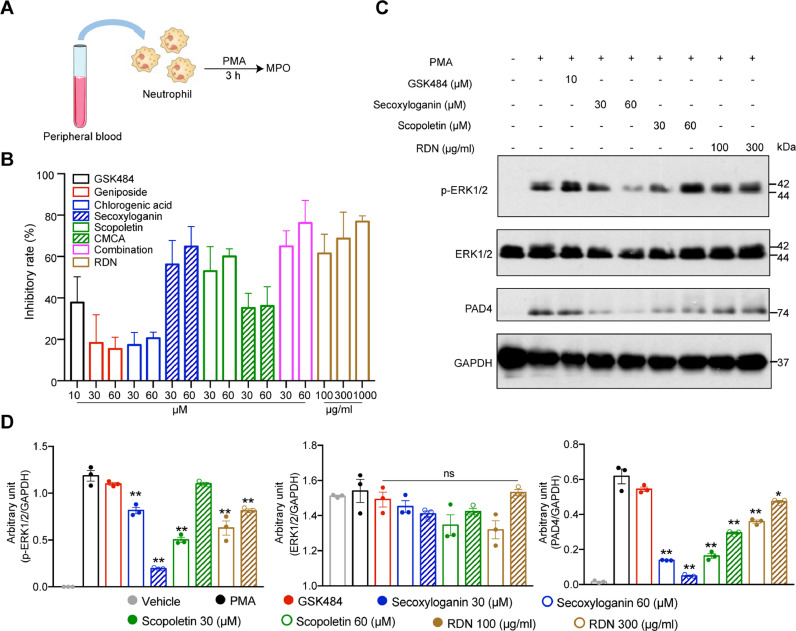
RDN injection suppressed the formation of NETs through MAPK pathway
PADI4 (PAD4) exists in downstream of ROS and calcium signaling during NETosis Papayannopoulos (2018). Thus, we speculated that RDN and their representative compounds may regulate the formation of NETs. To verify this hypothesis, we used PMA induced NETs formation, and then MPO was measured. Secoxyloganin from Lonicera japonica Thunb. and scopoletin from Artemisia annua L. obviously inhibited the expression of MPO in a dose-dependent manner (Fig. 7 A, B). As shown in Fig. 6C, RDN may inhibited the formation of NETs through MAPK pathway. We found that secoxyloganin suppressed the phosphorylation of ERK1/2 (Fig. 7C, D).
Fig. 7.
RDN injection reduced MAPK pathway in vitro. (A) Human neutrophils were stimulated with PMA (50 nM) for 3 hours. After 3 h, supernatants were collected (B) and the level of MPO was measured by using ELISA. (C) Western blot analysis of ERK 1/2, ERK 1/2 phosphorylation and PAD4. (D) The quantitative results of Western blot were shown. Data are shown as the mean ± SEM of three independent experiments. * p < 0.05, ** p < 0.01 vs. the PMA group.
Discussion
Traditional Chinese medicines are generally used clinically to treat various diseases and have significant effect (Gao et al., 2013). However, it is hard to clarify the molecular mechanism of Chinese medicines due to their muti-component and muti-target functions. The efficacy of RDN as an anti-pneumonia agent has been demonstrated. A large number of studies have shown that RDN can treat different types of lung injury by inhibiting MAPK, NF-κB and other signaling pathways (Huang et al., 2019; Jia et al., 2021; Jiang et al., 2019; Tang et al., 2014). In this study, we verified that RDN injection can ameliorate LPS-induced acute lung injury and the RDN group extend survival time. A large amount of experimental data shows that RDN inhibited the formation of NETs and suppressed the phosphorylation of ERK1/2.
Acute lung injury is characterized by pulmonary inflammation and impairment of the capillary endothelial barrier (Eckle et al., 2008; Matute-Bello et al., 2008; Wu et al., 2018). Previous studies demonstrated that ALI could escalate inflammation, including pro-inflammatory mediators (IL-1β, IL-6, TNF-α) and chemokines (Ma et al., 2015; Jiang et al., 2019) and further damage the alveolar-capillary barrier (Peng et al., 2016). In accord with these results, we found RDN injection decreased the protein and mRNA levels IL-1β, IL-6 and TNF-α in serum and lung tissue of mice. In addition, we used flow cytometry to detect the number of cells in BALF and found RDN can significantly suppress inflammatory cells infiltration such as T cell, macrophage and neutrophil, which was consistent with the improved histopathological result.
Neutrophils are one of important cells in ALI/ARDS. We found that RDN can significantly inhibit the production of neutrophils in vivo. We collected from TCMSP and DrugBank database. A total of 249 RDN-related targets were obtained. Then we collected from the GeneCards database and 1608 targets on ALI were obtained. Significantly, PAD4 is a common target between RDN and ALI. Peptidylarginine deiminase 4 (PAD4) can catalyze the transformation of arginine residues to citrulline in polypeptides. The formation of NETs needs PAD4-mediated deimination of histone H3 and H4 (Wang et al., 2004; Saskia et al., 2011; Li et al., 2010). Thus, we speculated that the inhibition of RDN in ALI may be related to NETs. NETs, consisted of modified cytotoxic granular proteins, are unique reticular structures released by neutrophils (Sollberger et al., 2018). NETs will be formed during ALI and ARDS and are related to disease progression and severity (Saffarzadeh et al., 2012; Cahilog et al., 2020). Previous study has illustrated a connection between extreme NETs formation and LPS-induced acute lung injury in mice (Khan et al., 2017), consistent with the result that acute respiratory distress syndrome patients appear higher levels of NETs in plasma and BALF (Lefrançais et al., 2018; Gray, 2018). In this study, we found that RDN inhibited the protein level of PAD4 in mouse lung tissue. Meanwhile, immunofluorescence results showed that RDN inhibited the expression of cit H3 and MPO. In addition, RDN suppressed the levels of NE and MPO in BALF, serum and lung tissue. GSK484 and Cl-amidine are PAD4 inhibitors, which can inhibit the formation of NETs. Therefore, we used them to treat acute lung injury, which reduce peribronchial and perivascular leukocyte infiltration. The above results indicate that an excessive NETs formation in LPS-induced acute lung injury.
KEGG enrichment analysis predicted that RDN is associated with MAPK pathway and thus influence the formation of NETs. The MAPK pathway includes p38, ERK1/2 and JNK, all of which have essential functions in lung injury (Chen et al., 2015). Previous studies demonstrated ROS-inducing receptors and kinases, such as MEK (MAPK/ERK kinase), ERK and PI3K, have been associated with PMA-induced the formation of NETs (Papayannopoulos, 2018; DeSouza-Vieira et al., 2016; Hakkim et al., 2011). Thus, we examined the effect of RDN on PMA-induced NETs formation in neutrophils. We selected six pharmacological activity of compounds from Lonicera Japonica Thunb., Gardenia Jasminoides Ellis and Artemisia Annua L. based on HPLC results and literature reviews. The results showed secoxyloganin from Lonicera Japonica Thunb., scopoletin from Artemisia Annua L. and RDN significantly suppressed the phosphorylation of ERK1/2 and PAD4. These results suggested that suppression of NETs formation might be involved in the treatment of RDN through MAPK pathway. It may reveal new light on the further mechanisms and the underlying material basis of RDN on ALI.
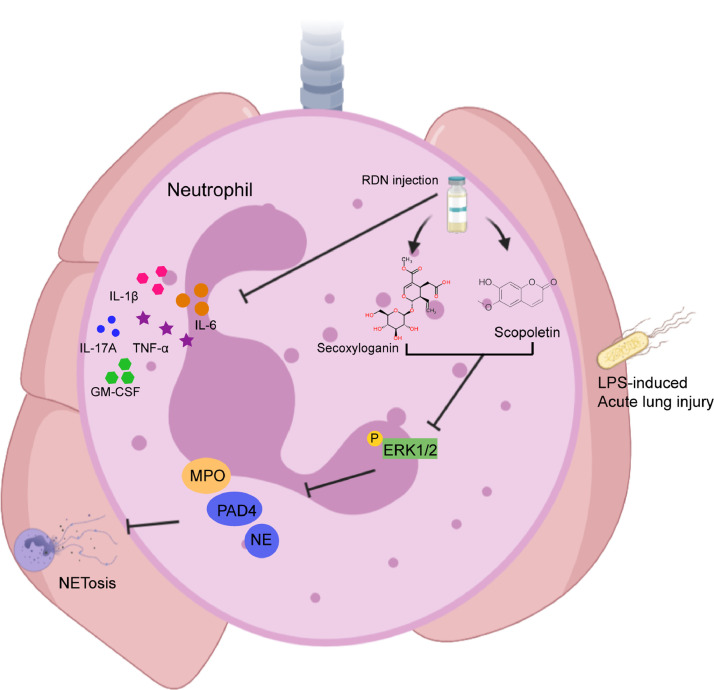
Conclusions
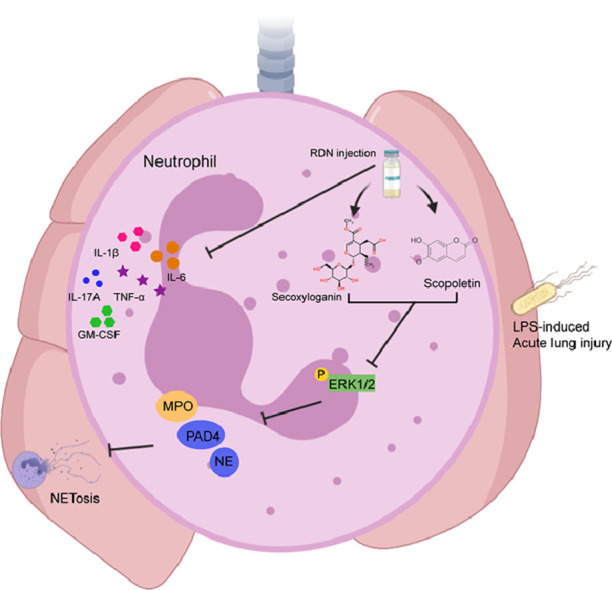
In conclusion, RDN inhibits the production of inflammatory cytokines and the formation of NETs via down-regulating MAPK pathway, thus alleviating LPS-induced acute lung injury (Fig. 8 ).
Fig. 8.
RDN relieved LPS-induced lung injury by inhibition of NETs formation through MAPK pathway. RDN and their compounds inhibit the phosphorylation of ERK1/2-related NETs formation, thus ameliorating LPS-induced acute lung injury.
CRediT authorship contribution statement
Chenxi Yang: Writing – original draft, Investigation, Formal analysis. Chenglin Song: Writing – original draft, Formal analysis. Yitong Liu: Methodology, Conceptualization. Jiao Qu: Conceptualization. Haibo Li: Resources. Wei Xiao: Resources. Lingdong Kong: Supervision. Huiming Ge: Supervision, Project administration, Funding acquisition. Yang Sun: Supervision, Project administration, Funding acquisition. Wen Lv: Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos. 81872877, 91853109), Center for Uterine Cancer Diagnosis & Therapy Research in Zhejiang Province (JBZX-201803)
References
- Cahilog Z., Zhao H., Wu L., Alam A., Eguchi S., Weng H., Ma D. The role of neutrophil NETosis in organ injury: novel inflammatory cell death mechanisms. Inflammation. 2020;43:2021–2032. doi: 10.1007/s10753-020-01294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Mou Y., Tan J., Wei L., Qiao Y., Wei T., Xiang P., Peng S., Zhang Y., Huang Z., Ji H. The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2015;25:55–64. doi: 10.1016/j.intimp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- DeSouza-Vieira T., Guimaraes-Costa A., Rochael N.C., Lira M.N., Nascimento M.T., Lima-Gomez P.S., Mariante R.M., Persechini P.M., Saraiva E.M. Neutrophil extracellular traps release induced by Leishmania: role of PI3Kgamma, ERK, PI3Ksigma, PKC, and [Ca2+] J. Leukoc Biol. 2016;100:801–810. doi: 10.1189/jlb.4A0615-261RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T., Grenz A., Laucher S., Eltzschig H.K. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Guo M., Peng L., Zhao B., Su J., Liu H., Zhang L., Bai X., Qiao Y. UPLC Q-TOF/MS-based metabolic profiling of urine reveals the novel antipyretic mechanisms of qingkailing injection in a rat model of yeast-induced pyrexia. Evid. Based Complement. Altern. 2013 doi: 10.1155/2013/864747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Huang C., Geng T., Chen X., Wang J., Liu J., Duan K., Cao L., Wang Z., Xiao W. Serum and urine metabolomics based on UPLC-Q-TOF/MS reveals the antipyretic mechanism of Reduning injection in a rat model. J. Ethnopharmacol. 2019;250 doi: 10.1016/j.jep.2019.112429. [DOI] [PubMed] [Google Scholar]
- Geng T., Si H.H., Kang D.Y., Li Y.J., Huang W.Z., Wang Z.Z., Bi Y., Zhang H., Xiao W. Influences of Re Du Ning Injection, a traditional Chinese medicine injection, on the CYP450 activities in rats using a cocktail method. J. Ethnopharmacol. 2015;174:426–436. doi: 10.1016/j.jep.2015.08.035. [DOI] [PubMed] [Google Scholar]
- Gong J., Wu Z.Y., Qi H., Chen L., Li H.B., Li B., Yao C.Y., Wang Y.X., Wu J., Yuan S.Y., Yao S.L., Shang Y. Maresin 1 mitigates LPS-induced acute lung injury in mice. Br. J. Pharmacol. 2014;171:3539–3550. doi: 10.1111/bph.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R.D. NETs in pneumonia: is just enough the right amount? Eur. Respir. J. 2018;51 doi: 10.1183/13993003.00619-2018. [DOI] [PubMed] [Google Scholar]
- Hakkim A., Fuchs T.A., Martinez N.E., Hess S., Prinz H., Zychlinsky A., Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- Huang X., Duan X., Zhu Y., Wang K., Wu J., Tian X. Comparative efficacy of Chinese herbal injections for the treatment of community-acquired pneumonia: A Bayesian network meta-analysis of randomized controlled trials. Phytomedicine. 2019;63 doi: 10.1016/j.phymed.2019.153009. [DOI] [PubMed] [Google Scholar]
- Jia S., Luo H., Liu X., Fan X., Huang Z., Lu S., Shen L., Guo S., Liu Y., Wang Z., Cao L., Cao Z., Zhang X., Zhou W., Zhang J., Li J., Wu J., Xiao W. Dissecting the novel mechanism of reduning injection in treating coronavirus disease 2019 (COVID-19) based on network pharmacology and experimental verification. J. Ethnopharmacol. 2021;273 doi: 10.1016/j.jep.2021.113871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Zhong R., Zhang J., Wang X., Ding G., Xiao W., Ma S. Reduning injection ameliorates paraquat-induced acute lung injury by regulating AMPK/MAPK/NF-kappaB signaling. J. Cell Biochem. 2019;120(8):12713–12723. doi: 10.1002/jcb.28540. [DOI] [PubMed] [Google Scholar]
- Jin S.W., Zhang L., Lian Q.Q., Liu D., Wu P., Yao S.L., Ye D.Y. Posttreatment with aspirin-triggered lipoxin A4 analog attenuates lipopolysaccharide-induced acute lung injury in mice: the role of heme oxygenase-1. Anesth. Analg. 2007;104:369–377. doi: 10.1213/01.ane.0000252414.00363.c4. [DOI] [PubMed] [Google Scholar]
- Khan M.A., Farahvash A., Douda D.N., Licht J.C., Grasemann H., Sweezey N., Palaniyar N. JNK activation turns on LPS- and gram-negative bacteria-induced NADPH oxidase-dependent suicidal NETosis. Sci. Rep. 2017;7:3409. doi: 10.1038/s41598-017-03257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançais E., Mallavia B., Zhuo H., Calfee C.S., Looney M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight. 2018;3:e98178. doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Li M., Lindberg M.R., Kennett M.J., Xiong N., Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Su X., Pan P., Zhang L., Hu Y., Tan H., Wu D., Liu B., Li H., Li H.S., Dai M., Li Y., Hu C., Tsung A. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci. Rep. 2016;6:37252. doi: 10.1038/srep37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yue Y., Pan P., Zhang L., Su X., Li H., Li H.S., Li Y., Dai M., Li Q., Mao Z. IRF-1 intervention in the classical ROS-dependent release of NETs during LPS-induced acute lung injury in mice. Inflammation. 2018;42(1):387–403. doi: 10.1007/s10753-018-0903-7. [DOI] [PubMed] [Google Scholar]
- Matthay M.A., Ware L.B., Zimmerman G.A. The acute respiratory distress syndrome. J. Clin. Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute-Bello G., Frevert C.W., Martin T.R. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhang X., Su Z., Li N., Cao L., Ding G., Wang Z., Xiao W. Insight into the molecular mechanism of a herbal injection by integrating network pharmacology and in vitro. J. Ethnopharmacol. 2015;173:91–99. doi: 10.1016/j.jep.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Mikacenic C., Moore R., Dmyterko V., West T.E., Altemeier W.A., Liles W.C., Lood C. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator-associated pneumonia. Crit. Care. 2018;22:358. doi: 10.1186/s13054-018-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- Peng S., Hang N., Liu W., Guo W., Jiang C., Yang X., Xu Q., Sun Y. Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-κB pathways. Acta Pharm Sin B. 2016;6:205–211. doi: 10.1016/j.apsb.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffarzadeh M., Juenemann C., Queisser M., Lochnit G., Barreto G., Galuska S.P., Lohmeyer J., Preissner K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLos One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saskia H., John R.T., Sanja A., Kerra A.M. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011;6(7):e22043. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger G., Tilley D.O., Zychlinsky A. Neutrophil extracellular traps: the biology of chromatin externalization. Dev. Cell. 2018;44:542–553. doi: 10.1016/j.devcel.2018.01.019. [DOI] [PubMed] [Google Scholar]
- Szapiel S.V., Elson N.A., Fulmer J.D., Hunninghake G.W., Crystal R.G. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am. Rev. Respir. Dis. 1979;120:893–899. doi: 10.1164/arrd.1979.120.4.893. [DOI] [PubMed] [Google Scholar]
- Tang L., Xiao W., Li Y., Li H., Wang Z., Yao X., Kurihara H., He R. Anti-inflammatory effects of reduning injection on lipopolysaccharide-induced acute lung injury of rats. Chin. J. Integr. Med. 2014;20:591–599. doi: 10.1007/s11655-014-1758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wysocka J., Sayegh J., Lee Y.H., Perlin J.R., Leonelli L., Sonbuchner L.S., McDonald C.H., Cook R.G., Dou Y., Roeder R.G., Clarke S., Stallcup M.R., Allis C.D., Coonrod S.A. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- Wu Y., He H., Nie Y., Ding Y., Sun L., Qian F. Protostemonine effectively attenuates lipopolysaccharide-induced acute lung injury in mice. Acta Pharmacol. Sin. 2018;39:85–96. doi: 10.1038/aps.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., Schneider A.H., Caetite D., Tavares L.A., Paiva I.M., Rosales R., Colon D., Martins R., Castro I.A., Almeida G.M., Lopes M.I.F., Benatti M.N., Bonjorno L.P., Giannini M.C., Luppino-Assad R., Almeida S.L., Vilar F., Santana R., Bollela V.R., Auxiliadora-Martins M., Borges M., Miranda C.H., Pazin-Filho A., da Silva L.L.P., Cunha L.D., Zamboni D.S., Dal-Pizzol F., Leiria L.O., Li S., Batah S., Fabro A., Mauad T., Dolhnikoff M., Duarte-Neto A., Saldiva P., Cunha T.M., Alves-Filho J.C., Arruda E., Louzada-Junior P., Oliveira R.D., Cunha F.Q. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020;217(12) doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]