Abstract
The reward system, which consists of dopaminergic neurons projecting from the ventral tegmental area (VTA) to the nucleus accumbens and caudate-putamen in the striatum, has an important role in the pathogenesis of not only drug addiction but also diet-induced obesity. In the present study, we examined whether signaling through glucocorticoid receptors (GRs) in the reward system affects the rewarding value of a high-fat diet (HFD). To do so, we generated mice that lack functional GRs specifically in dopaminergic neurons (D-KO mice) or corticostriatal neurons (CS-KO mice), subjected the mice to caloric restriction stress conditions, and evaluated the rewarding value of a HFD by conditioned place preference (CPP) test. Caloric restriction induced increases in serum corticosterone to similar levels in all genotypes. While CS-KO as well as WT mice exhibited a significant preference for HFD in the CPP test, D-KO mice exhibited no such preference. There were no differences between WT and D-KO mice in consumption of HFD after fasting or cognitive function evaluated by a novel object recognition test. These data suggest that glucocorticoid signaling in the VTA increases the rewarding value of a HFD under restricted caloric stress.
Subject terms: Feeding behaviour, Endocrine system and metabolic diseases, Reward
Introduction
Feeding behavior is known to be regulated by not only the hypothalamus but also the reward system1,2, in which dopaminergic neurons in the ventral tegmental area (VTA) and substantia nigra project to the nucleus accumbens (NAc) and caudate-putamen (CPu), respectively, in the striatum3. This neurocircuit is activated by consuming palatable food, processes information related to food reward, and provides emotional satiety4. Studies have also revealed that the reward system is involved in the pathophysiology of diet-induced obesity3,5,6, which is a major health issue in many countries7.
A high-fat diet (HFD), which consists of a highly palatable food, is suggested to have addiction-like properties such as cocaine or heroin8. There are several lines of evidence to support that rodents become more addicted to narcotic substances under stress conditions or after administration of glucocorticoids9,10. Stress also promotes consumption of palatable food11, and previous studies have shown that increases in serum cortisol concentrations in response to stress are closely correlated with the amount of snack intake in human subjects12, and that mice which were moderately food-restricted in advance showed a significant increase in binge eating of a palatable high-fat food during stress exposure13. These results suggest that glucocorticoid signaling activated by stress can stimulate the reward system and thereby increase intake of palatable food, although the detailed mechanisms have not yet been clarified.
In the present study, we generated mice that lack functional GRs specifically in dopaminergic and corticostriatal neurons, and examined whether signaling through GRs in the reward system affects the rewarding value of a HFD by using a conditioned place preference (CPP) test, in order to investigate the role of glucocorticoid signaling in the reward system.
Results
Generation of dopaminergic neuron-specific and corticostriatal neuron-specific GR-deficient mice
To generate dopaminergic neuron-specific GR deficient mice, GRloxP/loxP mice were crossed with dopamine transporter (DAT)-Cre (DAT-Cre) mice. We next crossed GR+/loxP DAT- Cre mice with GRloxP/loxP or GR+/loxP mice to yield GRloxP/loxP DAT-Cre (hereafter termed D-KO) mice and GRloxP/loxP littermate controls (hereafter termed WT) mice. GPR88 is an orphan G-protein-coupled receptor that is highly expressed in striatal and cortical neurons14,15. To generate corticostriatal neuron-specific GR-deficient mice, GRloxP/loxP mice were crossed with GPR88-Cre mice. We next crossed GR+/loxP GPR88-Cre mice with GRloxP/loxP or GR+/loxP mice to yield GRloxP/loxP GPR88-Cre (hereafter termed CS-KO) mice and GRloxP/loxP littermate controls (hereafter termed WT) mice. Deletion of the GR allele (Δ) in D-KO mice was only detected in DNA extracts from the VTA (Fig. 1A). Similarly, deletions of the GR allele (Δ) in CS-KO mice were detected in DNA extracts from the NAc and CPu (Fig. 1B). In contrast, no deletion of the floxed alleles was detected by PCR in WT mice (Fig. 1A,B). Immunostaining for both DAT and GR revealed that GRs were expressed in dopaminergic neurons of the VTA in WT mice, whereas GR-expressing cells in the VTA were rarely detected in D-KO mice (Fig. 1C). To assess the localization of GPR88 and GR, we crossed CS-KO mice to ROSA26 Cre-reporter knock-in mice (hereafter termed R26GRR), in which green fluorescence changed to red fluorescence in Cre-recombined cells16. GRloxP/loxP GPR88-Cre R26GRR mice expressed tdsRed-positive cells in the NAc and CPu, and GR immunostaining revealed that GR was expressed in the tdsRed-positive cells in the NAc and CPu in WT but not in CS-KO mice (Fig. 1D,E), while GR was expressed in the tdsRed-positive cells in mPfc and Ofc in WT as well as CS-KO mice (Supplementary Figure 1). The ratio of GR expressing cell number in the dopaminergic neurons in VTA or GPR88 expressing neurons in NAc was significantly decreased in D-KO mice (about 30%) or CS-KO (about 10%) mice compared with WT (Fig. 1F,G).
Figure 1.
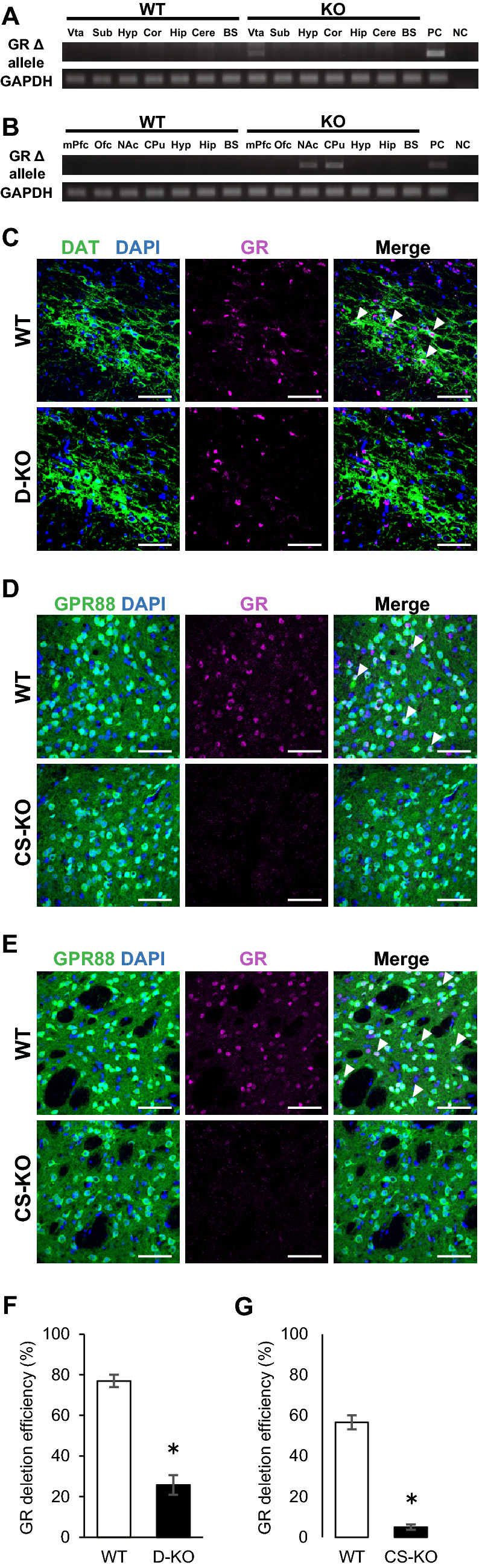
Generation of dopaminergic neuron-specific and corticostriatal neuron-specific GR deficient mice. (A,B) Detection of deletion of GR alleles (Δ) in DAT-GR-/- (D-KO) mice (A) and CPR88-GR-/- (CS-KO) mice (B) compared with GR+/+ mice (WT). Vta, ventral tegmental area; Sub, substantia nigra; Hyp, hypothalamus; Cor, cerebral cortex; Hip, hippocampus; Cer, cerebellum; BS, brainstem; mPfc, medial prefrontal cortex; Ofc, orbitofrontal cortex; NAc, nucleus accumbens; CPu, caudate-putamen; PC, positive control; NC, negative control. PCR reaction with GAPDH was used as an internal control. Deletion of the GR allele in D-KO mice was only detected in DNA extracts from the VTA (A). Similarly, deletions of the GR allele in CS-KO mice were detected in DNA extracts from the NAc and CPu (B). (C) Immunostaining of DAT and glucocorticoid receptor (GR) in the VTA of mice. Representative photographs show the staining of DAT (green), GR (red) and DAPI (blue) in the VTA in WT and D-KO mice. (D,E) Immunostaining of GR in the NAc (D) and CPu (E) of mice. Representative photographs show the staining of GR (red) and DAPI (blue) in NAc and CPu in GPR88-GR+/+ R26GRR mice (WT) and GPR88-GR-/- R26GRR mice (CS-KO). Scale bar: 50 µm. (F,G) The ratio of GR expressing cell number in the DAT expressing neurons in VTA (F) or GPR88 expressing neurons in NAc (G) (n = 4 for each group). All values are mean ± SEM. Statistical analyses were performed using unpaired t test. Asterisks denote significant group differences at p < 0.05.
Glucocorticoid signaling in the VTA increases the rewarding value of a HFD under caloric restriction stress
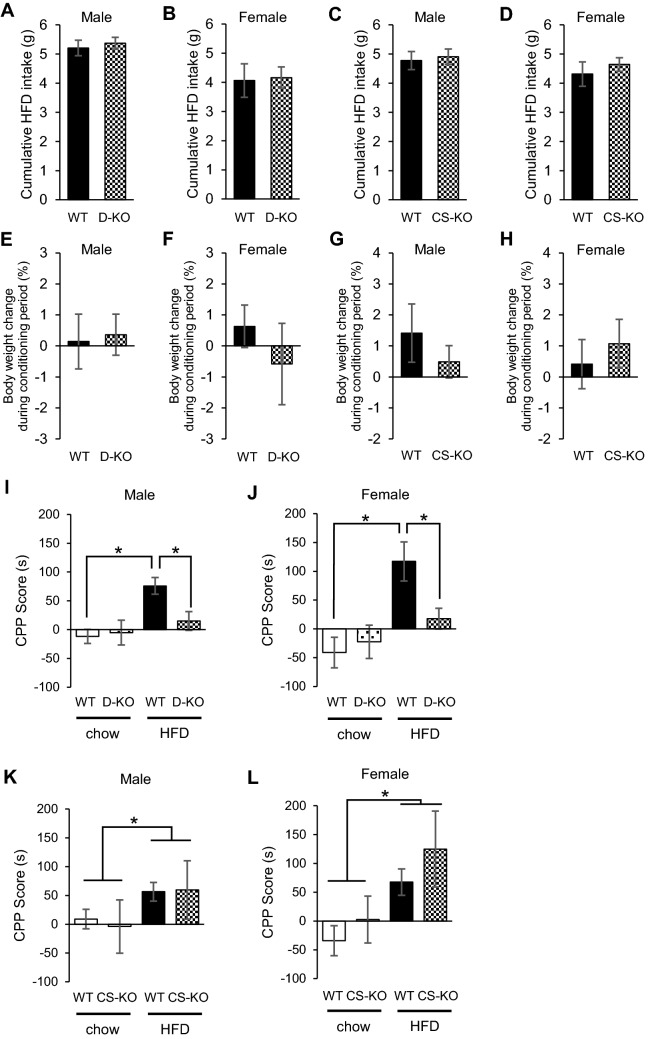
To investigate the role of glucocorticoid signaling in VTA, we performed experiments under stressed conditions in which corticosterone, a glucocorticoid hormone in mice, is expected to increase. We applied the CPP test, with which rewarding value of a palatable food can be evaluated17–19. Mice were placed on the protocol for caloric restriction (access to food only for 4 h during the light cycle) for 6 days prior to CPP preconditioning, and their BWs were reduced to 85%-90% of that at the start of the experiment. Caloric restriction was maintained throughout the session, so that mice underwent the experiment under a stressed condition. Both BW and cumulative intake of HFD were similar among WT, D-KO, and CS-KO mice under caloric restriction during the conditioning session (Fig. 2A–H). While WT and CS-KO mice exhibited a significant preference for the HFD [Male, effect of diet: F(1,39) = 3.935, p < 0.05; effect of genotype: F(1,39) = 0.008, p = 0.931; effect of interaction between diet and genotype: F(1,39) = 0.115, p = 0.737; Female, effect of diet: F(1,43) = 12.708, p < 0.01; effect of genotype: F(1,43) = 2.476, p = 0.123; effect of interaction between diet and genotype: F(1,43) = 0.287, p = 0.595 by two-way ANOVA followed by post hoc testing], D-KO mice exhibited no such preference [Male, effect of diet: F(1,43) = 12.014, p < 0.01; effect of genotype: F(1,43) = 3.292, p = 0.077; effect of interaction between diet and genotype: F(1,43) = 3,872, p < 0.05; Female, effect of diet: F(1,29) = 15.826, p < 0.01; effect of genotype: F(1,29) = 0.347, p = 0.561; effect of interaction between diet and genotype: F(1,29) = 7.119, p < 0.05 by two-way ANOVA followed by post hoc testing] (Fig. 2I–L). Conversely, preference for HFD was not observed in the CPP test between WT and D-KO mice under non-stressed condition (Supplementary Figure 2).
Figure 2.
Glucocorticoid signaling in VTA increases the rewarding value of a HFD under caloric restriction stress. (A–D) The amount of HFD consumed during CPP conditioning session. There were no differences between WT and D-KO (A: male n = 9–13, B: female n = 7–10 for each group), or CS-KO (C: male n = 8–14, D: female n = 8–14 for each group). (E–H) Body weight change during the CPP conditioning session. There were no differences between WT and D-KO (E: male n = 9–13, F: female n = 7–10 for each group), or CS-KO mice (G: male n = 8–14, H: female n = 8–14 for each group). (I–L) Results of the CPP test under caloric restriction stress. WT mice exhibited a significant preference for the HFD-paired compartment, while D-KO mice exhibited no preference for the HFD-paired compartment, in both males and females (I: male n = 9–13, J: female n = 7–10 for each group). Both WT and CS-KO mice exhibited a significant preference for the HFD-paired compartment, with no difference between genotypes (K: male n = 8–14, L: female n = 8–14 for each group). All values are mean ± SEM. Statistical analyses were performed using unpaired t test (A–H) or two-way factorial ANOVA (I–L) followed by Bonferroni post hoc test. Asterisks denote significant group differences at p < 0.05 for (I–L).
Glucocorticoid signaling in the VTA affects the mRNA expressions associated with dopamine signaling under caloric restriction stress
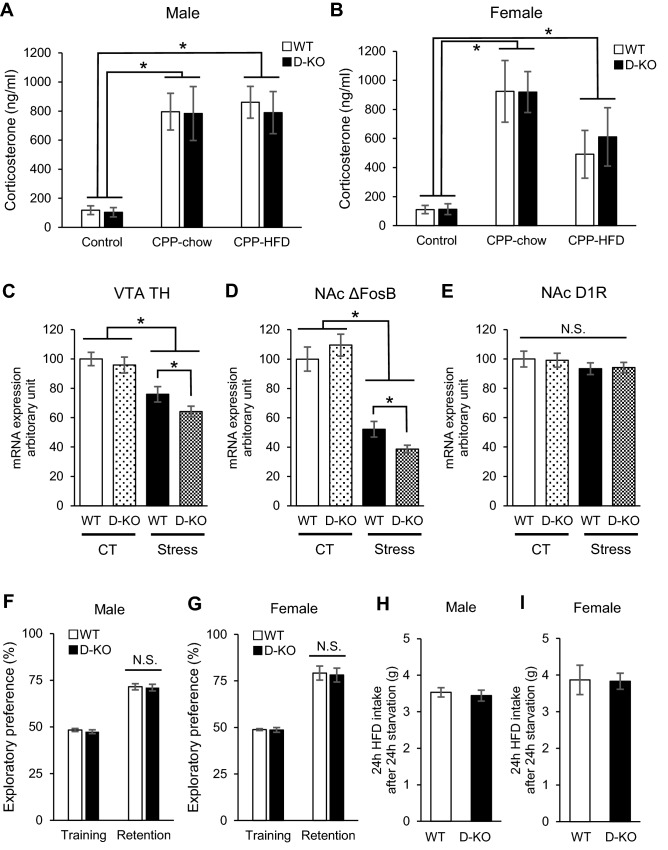
Serum levels of corticosterone were significantly increased in all groups subjected to the CPP test for 19 days compared with control mice, and there were no differences in serum corticosterone levels between genotypes [Male, effect of condition: F(2,36) = 22.829, p < 0.01; effect of genotype: F(1,36) = 0.113, p = 0.738; effect of interaction between condition and genotype: F(2,36) = 0.039, p = 0.962; Female, effect of condition: F(2,26) = 12.946, p < 0.01; effect of genotype: F(1,26) = 0.275, p = 0.604; effect of interaction between condition and genotype: F(2,26) = 0.083, p = 0.921 by two-way ANOVA followed by post hoc testing] (Fig. 3A,B). To investigate caloric restriction stress affects the mRNA expression levels associated with dopamine signaling in the mesolimbic system, we subjected female D-KO and WT mice into caloric restriction in which the mice could access to chow for 4 h per day during 19 days, and evaluated the changes of the mRNA expression levels of tyrosine hydroxylase (TH) in VTA, FBJ osteosarcoma oncogene B (ΔFosB) and dopamine1 receptor D1 (D1R) in NAc. In the control group that had no caloric restriction, there were no significant differences in the mRNA expression levels of TH, D1R and ΔFosB between genotypes (Fig. 3C–E). The caloric restriction reduced the mRNA expressions of TH and ΔFosB in both D-KO and WT mice compared to control groups (Fig. 3C,D), whereas there were no differences in D1R (Fig. 3E) [TH, effect of condition: F(1,28) = 38.775, p < 0.01; effect of genotype: F(1,28) = 3.516, p = 0.071; effect of interaction between diet and genotype: F(1,28) = 0.950, p = 0.338; ΔFosB, effect of condition: F(1,25) = 104.738, p < 0.01; effect of genotype: F(1,25) = 0.305, p = 0.586; effect of interaction between diet and genotype: F(1,25) = 4.934, p < 0.05 by two-way ANOVA followed by post hoc testing]. Of note, the mRNA expression levels of TH and ΔFosB in D-KO were significantly decreased compared to WT under caloric restriction (Fig. 3C,D). NORT was performed to evaluate the cognitive functions of mice. During the training session, both D-KO and WT mice spent equal amounts of time exploring either of the 2 objects and, thus, there was no biased exploratory preference in either group of animals (Fig. 3F,G). Total time spent in the exploration of a new object during the retention session did not differ between D-KO and WT mice (Fig. 3F,G). There were no significant differences in consumption of HFD after 24-h fasting between D-KO and WT mice (Fig. 3H,I). Similar results were obtained after fasting for 12 h or 48 h (data not shown).
Figure 3.
Caloric restriction affected the mRNA expressions of TH in VTA and ΔFosB in NAc via GR signaling in dopaminergic neurons, while corticosterone levels, cognitive function, and refeeding induced by caloric restriction stress showed no differences. (A,B) Serum corticosterone levels at the end of the CPP session, compared to control (under no stress). Both D-KO and WT exhibited significantly higher levels of serum corticosterone, with no difference between genotypes nor between feeding conditions (A: male, B: female, n = 5–7 for each group). (C–E) mRNA expressions of TH in VTA (C), ΔFosB (D) and D1R (E) in NAc after caloric restriction (termed ‘Stress’). In the control group, there were no significant differences in the mRNA expression levels between genotypes (C–E). The caloric restriction reduced the mRNA expressions of TH and ΔFosB in both D-KO and WT mice compared to control groups, with significant reduction in D-KO compared to WT (C,D) (female mice, n = 7–10 for each group). (F,G) NORT results. There were no significant differences in exploratory preference between D-KO and WT in both the training and retention sessions (F: male, G: female, n = 9–13 for each group). (H,I) HFD refeeding after fasting for 24 h. Both D-KO and WT, male (H) and female (I) mice exhibited no significant differences in the amount of HFD consumed (n = 5–7 for each group). All values are mean ± SEM. Statistical analyses were performed using two-way factorial ANOVA followed by Bonferroni post hoc test (A-G) or unpaired t test (H, I). Asterisks denote significant group differences at p < 0.05 vs control for (A-D).
Glucocorticoid signaling in dopaminergic and corticostriatal neurons does not affect energy metabolism
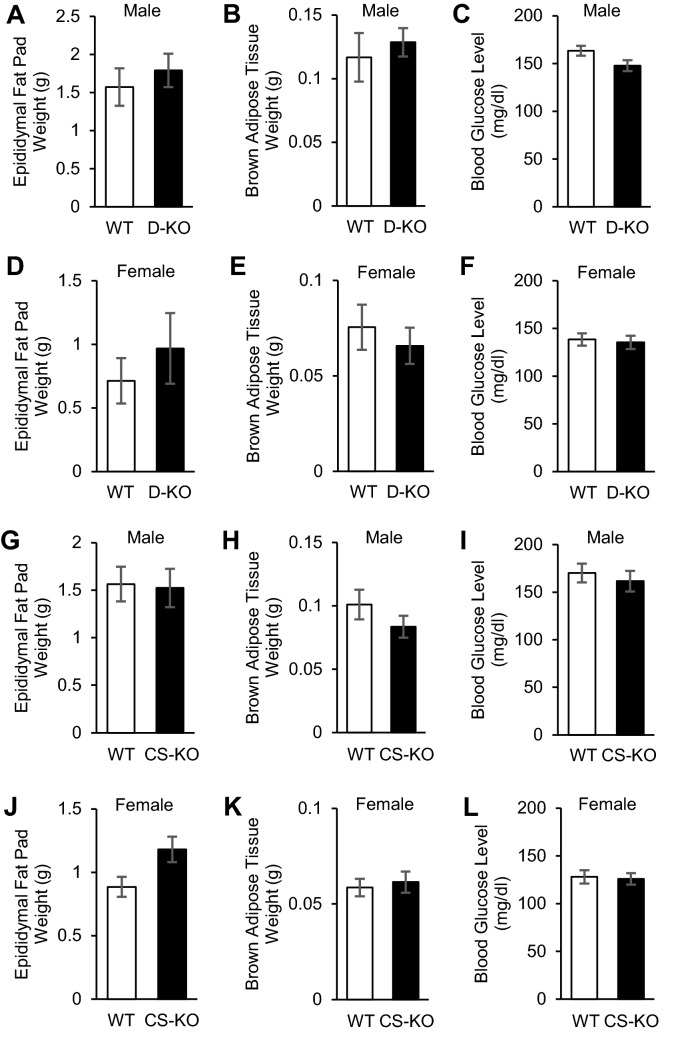
Finally, we examined the effects of glucocorticoid signaling in dopaminergic and corticostriatal neurons on energy metabolism. There were no significant differences in BW between WT, D-KO, and CS-KO mice with ad libitum access to HFD (Fig. 4A–D). There were no significant differences in epidydimal fat pad weight, brown adipose tissue weight, and blood glucose levels at the age of 16 weeks between WT, D-KO, and CS-KO mice fed HFD (Fig. 5) or fed chow (data not shown).
Figure 4.
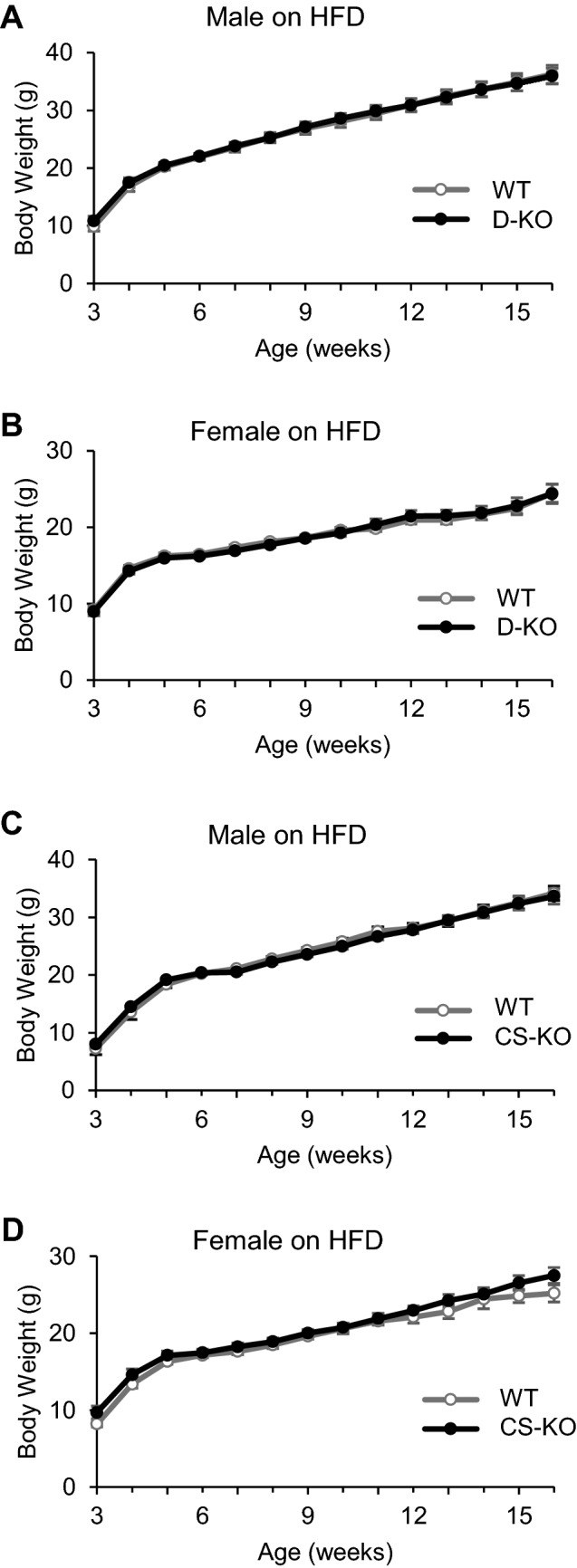
Glucocorticoid signaling in dopaminergic or corticostriatal neurons does not affect energy metabolism. (A,B) There was no significance between D-KO and WT mice for either male or female (n = 7–12 for each group). (C,D) There was no significance in body weight between CS-KO and WT mice for either male or female (n = 7–11 for each group). All values are mean ± SEM. Statistical analyses were performed using two-way ANOVA assessed for repeated measures.
Figure 5.
Body components and glucose metabolism were not affected by glucocorticoid receptor signaling in either dopaminergic neurons or corticostriatal neurons. (A–F) Epidydimal fat pad weights (A,D), brown adipose tissue weights (B,E), and blood glucose levels (C,F) of D-KO and WT mice at the age of 16 weeks (A–C, males, n = 7–9 for each: D–F, females, n = 7–12 for each). No significant differences in those parameters were found in either male or female mice. (G–L) Epidydimal fat pad weights (G,J), brown adipose tissue weights (H,K), and blood glucose levels (I,L) of CS-KO and WT mice at the age of 16 weeks (G-I, males, n = 9–11 for each: (J–L) females, n = 7–8 for each). No significant differences were found in those parameters in either male or female mice. All values are mean ± SEM. Statistical analyses were performed using unpaired t test.
Discussion
In the present study, a significant HFD preference in the CPP test was exhibited by WT and CS-KO mice but not by D-KO mice. Caloric restriction increased serum corticosterone levels similarly in WT and D-KO mice, and both cognitive function and the amount of HFD consumption after fasting were similar between genotypes. Taken together, it is suggested that glucocorticoid signaling in the VTA increases the rewarding value of a HFD under caloric restriction stress.
The CPP test employed in the present study is widely used to assess the rewarding value of a palatable food in murine models17–19. Although deletion of GRs in dopaminergic (D-KO mice) or corticostriatal (CS-KO mice) neurons had no effects on energy balance, D-KO but not CS-KO mice showed decreased preference for HFD compared with WT mice in the CPP test. Our data also showed that there was no difference in cognitive function between D-KO and WT mice, suggesting that the difference in CPP score between genotypes was not due to impairment of the acquisition or maintenance of memory. In addition, there were no significant differences in HFD consumption between D-KO and WT mice, suggesting that the motivation for eating was similar between genotypes. Taken together, it appears GR signaling in dopaminergic neurons potentiates the rewarding value of a palatable food.
HFD is generally considered to be a highly palatable food and is preferentially overconsumed20. HFD can stimulate the brain reward system independently of its caloric value, and the motivation to consume this palatable food can be high even in the absence of homeostatic energy requirements2. It is also reported that food-evoked dopamine release was amplified in food-deprived animals21, and that lowering glucose concentration increased glutamatergic miniature excitatory postsynaptic currents onto VTA dopamine neurons22. In addition, glucocorticoids are reported to modify postsynaptic dopaminergic transmission in the dorsolateral striatum23, and the gene for tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine biosynthesis, reportedly possesses a transcriptional-stimulatory glucocorticoid response element (GRE)-like sequence (GGCACAGTGTGGTCT) located in the 5’ flanking DNA between positions -2435 and -2421 from the transcription start site24. Furthermore, in the present study we found that the mRNA expression levels of TH in VTA and ΔFosB in NAc in D-KO mice were significantly decreased compared to WT mice under caloric restriction without changing the mRNA expression of D1R in NAc, suggesting that the activity of dopaminergic neurons was attenuated due to the lack of GR in VTA. Thus, it is possible that increased corticosterone under calorie-restricted conditions enhances the activity of dopaminergic neurons in the VTA through the GRE, although further study is necessary to prove this. Conversely, our data showed that the deletion of the GR in corticostriatal neurons did not affect the rewarding value of HFD. Consistent with our findings, a previous study reported that GRs in dopaminoceptive neurons did not affect the score on the CPP test, in which the preference for chocolate with cereals was measured under caloric restriction by using mice that lack the GR encoded by the Nr3c1 gene specifically for dopamine receptor 1a18.
Consistent with our previous study25, we detected recombination of the GR floxed allele only in VTA, suggesting that the D-KO mouse used in the present study is a model animal in which GR is selectively deleted in VTA. On the other hand, it is still unclear from this study whether GR deficiency in VTA affected the neural activity of dopaminergic neurons, as we did not evaluate dopamine concentrations. This is a limitation and further studies are required to clarify how glucocorticoids affect the dopaminergic neuronal system in the VTA.
In conclusion, glucocorticoid signaling in dopaminergic neurons of the VTA potentiates the rewarding value of a HFD under restricted caloric conditions.
Methods
Mice
All animal procedures were approved by the Animal Care and Use Committee of Nagoya University Graduate School of Medicine and performed in accordance with the institutional guidelines that conform to the National Institutes of Health animal care guidelines. The study was carried out in compliance with the ARRIVE guidelines. Mice were maintained on a 12-h light (9:00–21:00) /12-h dark cycle (21:00–9:00) in a temperature-controlled barrier facility, with free access to water and food. Age-matched littermates were used for all experiments.
Mice with DAT-specific and GPR88-specific deletion of GR
GRloxP/loxP mice previously generated by Dr. Günther Schütz26 were provided by EMMA(ID 02,124). DAT-Cre transgenic mice, in which the locus of Cre-recombinase is in the 3’ untranslated region of DAT gene27 (hereafter termed DAT-Cre mice), were provided by Jackson Laboratory (JAX stock #006,660). GPR88-Cre transgenic (RRID:IMSR_RBRC10287) mice (hereafter termed GPR88-Cre mice) express functional Cre-recombinase mainly in medium spiny neurons and a small population of parvalbumin-positive interneurons in the caudate-putamen and NAc14,15. ROSA26 Cre-reporter knock-in C57BL/6 N mice (RBRC 04,874), which exhibit green emission before and red after Cre-mediated recombination16 (hereafter termed R26GRR), were provided by RIKEN BRC through the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science and Technology, Japan. Primer sequences used for genotyping are shown as Table 1. DNA was extracted from the tail of each experimental mouse at the age of 10 days to check for the occurrence of a spurious germline deletion using the primers of GRΔ/Δ and GAPDH (for an internal control). All GRloxP/loxP mice, DAT-Cre mice, GPR88-Cre mice, and R26GRR mice were backcrossed more than 10 generations onto a C57BL/6 background.
Table 1.
Primers used in the present study.
| Gene | Forward primer (5’ → 3’) | Reverse primer (5’ → 3’) |
|---|---|---|
| GRloxP/loxP | GGCATGCACATTACTGGCCTTCT | CCTTCTCATTCCATGTCAGCATGT |
| DAT-Cre | TGGCTGTTGGTGTAAAGTGG |
GGACAGGGACATGGTTGACT [to detect wild-type (WT) gene] |
|
CCAAAAGACGGCAATATGGT (to detect transgene) | ||
| GPR88-Cre | ACCTGATGGACATGTTCAGGGATCG | TCCGGTTATTCAACTTGCACCATGC |
| R26GRR | AAAGTCGCTCTGAGTTGTTAT | CTTGTACAGCTCGTCCATGCCGAG |
| GRΔ/Δ | GGCATGCACATTACTGGCCTTCT | GTGTAGCAGCCAGCTTACAGGA |
| TH | TTGAAGGAACGGACTGGCTT | GAAACACACGGAAGGCCAGA |
| ΔFosB | AGGCAGAGCTGGAGTCGGAGAT | GCCGAGGACTTGAACTTCACTCG |
| D1R | GTAGCCATTATGATCGTCAC | GATCACAGACAGTGTCTTCAG |
| GAPDH (control) | AACGACCCCTTCATTGAC | TCCACGACATACTCAGCAC |
Isolating DNA from tissues for detection of recombination of floxed alleles
Tissues of mice at the age of 8 weeks were digested with 50 mM NaOH for 10 min at 95 °C, and 1 M Tris–HCl (pH 8.0) was added to the digestion. Samples were centrifuged for 10 min at 12,000 g and supernatants were transferred to a fresh tube. DNA was subjected to genotyping analyses by PCR with KOD FX DNA polymerase (Toyobo, Osaka, Japan) and the oligonucleotide primers. The PCR was performed with SimpliAmp Thermal Cycler (Thermo Fisher Scientific, MA, USA). The condition was 5 min at 95℃ followed by 30 cycles at 95℃ for 30 s, 56℃ for 20 s and 72℃ for 60 s with a 7 min final extension. After mixed with 6 × loading dye (Toyobo), the amplified products were electrophoresed with 1.5% agarose gel (Thermo Fisher Scientific) containing ethidium bromide (Merck Millipore, Darmstadt, Germany). DNA bands were visualized under ultraviolet light using Quantity-One software (Bio-Rad, CA, USA).
Body composition and measurement of BW
At weaning (3 weeks old), mice were placed on diets of either standard chow CE-2 (chow); calories provided by protein (24.9%), fat (4.6%), and carbohydrate (70.5%) (CLEA, Tokyo, Japan) or a custom HFD (58Y1); calories provided by protein (18.3%), fat (60.9%), and carbohydrate (20.1%) (Test diet). The measurement of BW in HFD-fed mice was performed until the age of 16 weeks, when mice were sacrificed. Epididymal fat pat weight, brown adipose tissue weight, and blood glucose levels were measured at the beginning of the light cycle (between 09:00 and 10:00), when mice were in the fed state.
Measurement of serum corticosterone levels
Blood was collected via submandibular bleeding from mice. Serum was separated by centrifugation at 6000 rpm for 10 min and corticosterone levels were measured by enzyme-linked immunosorbent assay (AssayPro, MO, USA).
Brain collection for immunohistochemistry
Mice were deeply anesthetized and transcardially perfused with a cold fixative containing 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) pH 7.4, between 09:00 and 10:00 in the fed state. After fixation, brains were removed and immersed in the same fixative for 2 h at 4 °C. The brains were kept in PBS containing 10%-20% sucrose at 4 °C for cryoprotection. They were embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Tokyo, Japan) and stored at -80 °C until sectioning. Brains were cut into 20-μm sections on a cryostat at -20 °C, thawed and mounted on Superfrost Plus microscope slides (Matsunami, Tokyo, Japan), and stored at -80 °C until immunohistochemistry was performed as described previously28.
Immunohistochemistry
The frozen sections were washed with PBS, 0.3% Triton X-100 in PBS (15 min) and 50 mM glycine (15 min) followed by blocking with a mixture of 3% bovine serum albumin (Wako, Osaka, Japan) in PBS for 1 h at room temperature. Next, the sections were incubated with anti-DAT antibody (1:500; Merck Millipore) or anti-GR (1:500; Santa Cruz Biotechnology, CA, USA) overnight at 4 °C. The sections were then treated with Alexa Fluor 488-conjugated anti-rat IgG secondary antibody (1:1,000; Thermo Fisher Scientific), Alexa Fluor 594-conjugated anti-rabbit IgG secondary antibody (1:1,000; Thermo Fisher Scientific) or Alexa Fluor 647-conjugated anti-rabbit IgG secondary antibody (1:1,000; Thermo Fisher Scientific), and DAPI (1:1000; Dojindo, Kumamoto, Japan) for 1 h at room temperature. After washing in 1 × PBS, sections were placed on slides, air dried, and cover slipped with Vectashield (Vector Labs, CA, USA). All fluorescently stained sections were examined with a confocal laser microscope (TiEA1R; Nikon Instech, Tokyo, Japan) and viewed using NIS-Elements software (Nikon Instech).
Caloric restriction and conditioned place preference test
Animals used in this experiment consisted of 14–16 week-old KO and WT mice fed with chow. As shown in Supplementary Figure 1, mice underwent a 19-day protocol consisting of caloric restriction, limited access to HFD, and CPP. All the experiments in this session were performed using caloric-restricted mice that were provided ad libitum access to chow or HFD for a continuous 4-h period daily between 15:00–20:30, and this caloric restriction was maintained throughout the protocol. From day 4 to 8, mice were fed HFD for 2 h followed by chow for 2 h to avoid neophobia and to induce addiction to HFD. The conditioned place preference test was performed according to previously reported methods17,29. The apparatus consisted of 2 compartments: a white Plexiglas box and a black Plexiglas box (16 × 16 × 7 cm). To enable a mouse to distinguish the boxes, the floor of the white box was covered with uneven Plexiglas, and that of the black box, with smooth black Plexiglas. The place conditioning schedule consisted of 3 phases: preconditioning, conditioning, and testing. In the preconditioning phase, each mouse was allowed to move freely between the boxes for 20 min once a day during 2 days (day 7 and 8). On day 8, the time spent in each of the boxes was measured using a MED-PC IV (Neuroscience Idea, Osaka, Japan). Conditioning was counterbalanced between compartments so as to ensure the procedure was unbiased. Conditioning sessions were performed on days 9 to 18. On even days, each mouse was confined to 1 conditioning compartment in the presence of HFD pellets for 30 min. On odd days, each mouse was confined to the other conditioning compartment in the presence of a chow pellet for 30 min. After conditioning, each mouse was fed chow for 3.5 h in their home cage every day. On day 19, the testing phase was performed similarly to the preconditioning phase, with free access to both compartments for 20 min, and the time spent in each of the boxes was measured. Conditioned place preference was calculated for each mouse as the difference in time spent in the HFD compartment between the preconditioning and testing sessions. The control mice underwent the same protocol with chow instead of HFD throughout the protocol.
Analysis of mRNA expression levels
The VTA and NAc of WT and D-KO mice were dissected from mice fed a chow or mice with caloric restriction. Caloric restriction in this session were performed based on the same protocol as CPP, by which mice were provided ad libitum access to chow for a continuous 4-h period daily between 16:00–20:00 for 19 days. Before dissection, mice were deeply anesthetized using 2.0% isoflurane with an animal anesthetizer device (MK-AT210; Muromachi Kikai, Tokyo, Japan). Immediately after removing, the brain tissue was stored at − 80 °C until analysis. Total RNA was extracted from samples using TRIzol (Thermo Fisher Scientific) and the RNeasy kit (QIAGEN, Hilden, Germany). cDNA was synthesized from 100 ng total RNA using the ReverTra Ace qPCR RT Kit (Toyobo). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies, Santa Clara, CA), and samples were run using the CFX Connect Real-Time PCR Detection System (Bio-Rad). The relative mRNA levels of TH (tyrosine hydroxylase) in VTA, Fosb (FBJ osteosarcoma oncogene B) and Drd1 (dopamine receptor D1) in NAc were assessed by qRT-PCR using Gapdh as an internal control. Relative mRNA expression levels were calculated using the comparative Ct method as described previously28. Primer sequences are listed in Table 1.
Novel object recognition test
The novel object recognition test (NORT) was performed according to previously reported methods30,31. The experimental apparatus consisted of a Plexiglas open-field box (30 × 30 × 35 high cm), the floor of which was covered in sawdust. The apparatus was located in a sound-attenuated room and was illuminated at an intensity of 15 Lux. Shown in Supplementary Figure 4, the NORT procedure consisted of 3 sessions: habituation, training, and retention sessions. Each mouse was individually habituated to the box, with 10 min of exploration in the absence of objects each day for 3 consecutive days (habituation session, days 1–3). During the training session, 2 novel objects were symmetrically fixed to the floor of the box, 8 cm from the walls, and each animal was allowed to explore in the box for 10 min (day 4). The objects were constructed from a golf ball, wooden column, and wall socket, which were different in shape and color but similar in size. The animals were considered to be exploring the object when the head of the animal was facing the object or the animal was touching or sniffing the object. The time spent exploring each object was recorded. During the retention sessions, the animals were placed back into the same box 24 h (day 5) after the training session, in which one of the familiar objects used during training was replaced by a novel object. The animals were then allowed to explore freely for 5 min and the time spent exploring each object was recorded. Throughout the experiments, the objects were used in a counterbalanced manner in terms of their physical complexity and emotional neutrality. A preference index, a ratio of the amount of time spent exploring any 1 of the 2 objects (training session) or the novel object (retention session) over the total time spent exploring both objects, was used to measure cognitive function.
Statistical analysis
Statistical significance of the differences between groups was analyzed by either unpaired t test, two-way factorial ANOVA or two-way ANOVA with repeated measures followed by Bonferroni’s test. Results are expressed as mean ± SEM and differences were considered statistically significant at p < 0.05.
Supplementary Information
Acknowledgements
We thank Michiko Yamada for helpful technical assistance. A. Mizoguchi and R. Banno contributed equally to this work.
Author contributions
AM, RB, TN and HA designed the study and wrote the manuscript. AM, RS, HY, KT, MS, TT, HT and YI performed the experiments and analyzed the data with technical help and advice from RB, TK, TO, DH, SI, HS, TN, KY and HA.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ryoichi Banno, Email: ryouichi@med.nagoya-u.ac.jp.
Hiroshi Arima, Email: arima105@med.nagoya-u.ac.jp.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-92386-7.
References
- 1.Ferrario CR, et al. Homeostasis meets motivation in the battle to control food intake. J. Neurosci. 2016;36:11469–11481. doi: 10.1523/JNEUROSCI.2338-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denis RGP, et al. Palatability can drive feeding independent of AgRP neurons. Cell Metab. 2015;22:646–657. doi: 10.1016/j.cmet.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Der Klaauw AA, Farooqi IS. The hunger genes: pathways to obesity. Cell. 2015;161:119–132. doi: 10.1016/j.cell.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Obesity and overweight. (2018). Available at: http://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
- 8.Epstein DH, Shaham Y. Cheesecake-eating rats and the question of food addiction. Nat. Neurosci. 2010;13:529–531. doi: 10.1038/nn0510-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miczek KA, Nikulina EM, Shimamoto A, Covington HE. Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J. Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graf EN, et al. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J. Neurosci. 2013;33:11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ip CK, et al. Amygdala NPY circuits promote the development of accelerated obesity under chronic stress conditions. Cell Metab. 2019;30:111–128.e6. doi: 10.1016/j.cmet.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Newman E, O’Connor DB, Conner M. Daily hassles and eating behaviour: the role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32:125–132. doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J. Neurosci. 2010;30:16399–16407. doi: 10.1523/JNEUROSCI.1955-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hisatsune C, Ogawa N, Mikoshiba K. Striatum-specific expression of Cre recombinase using the Gpr88 promoter in mice. Transgenic Res. 2013;22:1241–1247. doi: 10.1007/s11248-013-9711-x. [DOI] [PubMed] [Google Scholar]
- 15.Quintana A, et al. Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue-dependent behaviors. Nat. Neurosci. 2012;15:1547–1555. doi: 10.1038/nn.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa Y, et al. Novel ROSA26 cre-reporter knock-in C57BL/6N Mice exhibiting green emission before and red emission after cre-mediated recombination. Exp. Anim. 2013;62:295–304. doi: 10.1538/expanim.62.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang JC, et al. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Invest. 2011;121:2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parnaudeau S, et al. Glucocorticoid receptor gene inactivation in dopamine-innervated areas selectively decreases behavioral responses to amphetamine. Front. Behav. Neurosci. 2014;8:1–12. doi: 10.3389/fnbeh.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perello M, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol. Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berthoud H-R, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. AJP Regul. Integr. Comp. Physiol. 2011;300:R1266–R1277. doi: 10.1152/ajpregu.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008;156:865–871. doi: 10.1016/j.neuroscience.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng Z, Santiago AM, Thomas MP, Routh VH. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol. Cell. Neurosci. 2014;62:30–41. doi: 10.1016/j.mcn.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrot M, et al. Influence of glucocorticoids on dopaminergic transmission in the rat dorsolateral striatum. Eur. J. Neurosci. 2001;13:812–818. doi: 10.1046/j.1460-9568.2001.01434.x. [DOI] [PubMed] [Google Scholar]
- 24.Hagerty T, Morgan WW, Elango N, Strong R. Identification of a glucocorticoid-responsive element in the promoter region of the mouse tyrosine hydroxylase gene. J. Neurochem. 2001;76:825–834. doi: 10.1046/j.1471-4159.2001.00072.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsunekawa T, et al. GABAB receptor signaling in the mesolimbic system suppresses binge-like consumption of a high-fat diet. iScience. 2019;20:337–347. doi: 10.1016/j.isci.2019.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 27.Bäckman CM, et al. Characterization of a mouse strain expressing Cre recombinase from the 30 untranslated region of the dopamine transporter locus. Gensis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- 28.Tsunekawa T, et al. Deficiency of PTP1B attenuates hypothalamic inflammation via activation of the JAK2-STAT3 pathway in microglia. EBioMedicine. 2017;16:172–183. doi: 10.1016/j.ebiom.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagai T, et al. The rewards of nicotine: regulation by tissue plasminogen activator-plasmin system through protease activated receptor-1. J. Neurosci. 2006;26:12374–12383. doi: 10.1523/JNEUROSCI.3139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang YP, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 31.Kamei H, et al. Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol. Psychiatry. 2006;59:75–84. doi: 10.1016/j.biopsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.