Abstract
Bioethanol has been considered as a more sustainable alternative for fossil fuels, and it has been used as a drop-in fuel mixture. In this paper, the autoxidation properties of real kerosene as well as single, binary and ternary surrogates with the presence of ethanol are investigated for the first time. A simplified python code is proposed to predict the pressure drop of the PetroOXY method that was used for assessing the fuel autoxidation properties. The experimental results show that the addition of an ethanol concentration reduces the induction period of real kerosene while increasing that of surrogate mixtures. Also, the maximum pressure during the PetroOXY test increases with the increase of ethanol concentration. The model is able to predict the induction period of ethanol accurately by employing an automated reaction mechanism generator. A strategy to increase the autoxidation stability of ethanol by adding 1 g/L antioxidant has been evaluated. The efficiency of the antioxidants for ethanol is in the following order: PY > Decalin > DTBP > Tetralin > BHT > MTBP > BHA > TBHQ > PG.
Keywords: Ethanol, Kerosene, Autoxidation, PetroOXY
Highlights
-
•
Autoxidation of real and surrogates kerosene was evaluated using PetroOXY method.
-
•
Ethanol addition decreases the induction period of real kerosene while increases that of surrogates.
-
•
Nine antioxidants were assessed to improve the thermal stability of ethanol.
-
•
A new method for modelling PetroOXY test is proposed.
Ethanol; Kerosene; Autoxidation; PetroOXY
1. Introduction
During storage to engine operation, fuel chemical and physical properties may change due to autoxidation by exposure to a higher temperature, pressure, or concentration of oxygen (Batts and Fathoni, 1991; Arun et al., 2020). The change of fuel viscosity, energy content as well as gums and particulates formation cause deficiency of engines fuel system and combustion. Autoxidation is more susceptible in jet engines since the fuel acts as a coolant, and thus the fuel stability is regulated in ASTM D1655.
Commercial and military jet fuels are mostly kerosene-based and derived from crude oil distillation. The environmental impact from burning fossil fuels promotes the utilisation of biofuels, such as bioethanol (Nikkhah et al., 2020; Sadhukhan et al., 2019). However, the direct use of alcohol has not yet been approved and requires conversion to alcohol-to-jet synthetic paraffinic kerosene (ATJ SPK) following ASTM D1655 (Buffi et al., 2017; Braz and Mariano, 2018). Alcohol fuels have different chemical and physical properties compared to aviation kerosene that might cause atomisation and combustion problems in the engine (Rao et al., 2017). Bioethanol has been implemented for diesel (Shahir et al., 2015) and gasoline (Chansauria and Mandloi, 2018; Dhande et al., 2021) alternatives while studies have started investigating the impact of ethanol addition to kerosene combustion characteristics.
An experimental study of a kerosene/ethanol blend in a cylindrical combustor was performed by Patra et al. (2015) which investigated the flame brightness, temperature of the combustor wall, CO, and CO2 concentration at the exhaust. The combustion characteristics of Jet A-1/ethanol flame droplets have been investigated by Rao et al. (2017) for different ethanol concentration up to 50 per cent. Unlike the individual fuels, the blend indicated a disruptive nature, such as puffing, micro and abrupt explosion. An experimental study of the atomisation of kerosene/ethanol fuel in an acoustic field was performed by Ju et al. (2017) using high-speed imaging, which found that the influence of the acoustic field being larger for ethanol than kerosene, and this was because of the smaller size of the ethanol droplet. An experimental study of the spray characteristics of ethanol-blended jet fuel was conducted by Song et al. (2016) with a high-pressure common-rail injection system. This study was focused on the penetration of the spray tip, cone angle, area, and concentration distribution of the spray. The comparison between several ethanol concentrations up to 30 per cent shows that 20 per cent of ethanol blend demonstrated ideal spray characteristics of an aviation gas turbine.
The trend of using biofuel as a drop-in fuel has motivated researchers to study the stability of the blend. Karavalakis et al. (2010) studied the stability of diesel/biodiesel blend using the Rancimat method, which shown the increase of blended fuel instability with the increasing biodiesel concentration. Also, the stability characteristics of the biodiesel samples vary since it was derived from various animal fats and vegetable oils, which results in different compositions and chemical structures. To improve the stability of biodiesel, the antioxidants addition effect on biodiesel stability has been studied. Zhou et al. (2016) investigated the performance of four different antioxidants with different concentrations to enhance biodiesel stability. The study indicated that each antioxidant has a unique impact on biodiesel stability. Ryu (2010) investigated the antioxidants addition effects on the oxidation stability of soybean biodiesel fuel, the performance and the exhaust emissions of a diesel engine. The study found that the antioxidant addition had minor effects on the engine emission and exhaust emission while improved the fuel stability. With the further development of biodiesel resources, a study on biodiesel stability test and strategy to improve its stability has been proposed (Saluja et al., 2016).
Studies of jet fuel autoxidation have resulted in the understanding of the chemistry as well as the availability of the kinetic models. The early kinetic modelling study of jet fuel autoxidation was performed by Zabarnick (1993), which provides a kinetic model for jet fuel and antioxidant. However, this study is lacking experimental validation to verify the proposed rate parameters and the jet fuel was modelled as a single bulk fuel species, RH. More recent studies have updated the reaction rate parameters of the model as well as the validation against experimental data. Liu et al. (2019) updated the reaction mechanism of jet fuel autoxidation, and it has been accurately validated with the deposition formation from their experiment. An accurate kinetic model provides detailed insight and identification of important parameters of the autoxidation process for fuel design optimisation (Malani et al., 2021).
Considering that real kerosene consists of hundreds of hydrocarbon species, studies have formulated surrogate mixtures to simplify the kinetic model. Humer et al. (2007) proposes a surrogate mixture that consists of n-alkane, cycloalkanes, and aromatics with a volumetric percentage of 60, 20, and 20 per cent, respectively. The n-alkane component is represented by n-decane or n-dodecane, while the cycloalkane component is represented by methylcyclohexane, and the aromatic component is represented by toluene or o-xylene. This mixture was relatively accurate in reproducing the real ignition characteristics of real aviation kerosene in a counter-flow diffusion flame experiment. Also, the proposed model that used the surrogate mixture was able to predict the experimental data from real kerosene. Honnet et al. (2009) proposed a mixture of 80% n-decane and 20% trimethyl benzene to model the real kerosene composition. Using this surrogate, the proposed model and combustion characteristics of the mixture were able to replicate the experimental data of strain rate extinction, autoignition, and soot volume fraction from a counter-flow diffusion flame of real kerosene. However, none of these surrogate models has been evaluated to represent the liquid-phase oxidation of real kerosene.
Kinetic models for jet fuel surrogate components are available in the literature, including experimental data validation. Mielczarek (2015) used Reaction Mechanism Generator (RMG) (Gao et al., 2016) and a pressure drop model for simulating the autoxidation of n-dodecane and n-decane as well as the validation with PetroOXY pressure drop. It was found that the model could accurately simulate the pressure drop of n-decane and n-dodecane from the PetroOXY test. The initial oxygen concentration for the modelling work was obtained from an approximation using Henry's Law which the initial oxygen concentration value in the liquid phase was similar for all solvents. However, the initial oxygen concentration should vary with different fuel samples as it has different Henry constants and maximum pressure during the PetroOXY test, which affects the initial oxygen concentration. Ben Amara et al. (2013) studied n-dodecane autoxidation in a Rancimat experiment and proposed a reaction mechanism that can accurately represent the induction period data from the experiment. de Jesus Silva et al. (2020) studied the kinetics of thermal degradation of poly(vinylidene fluoride) in bioethanol, and it was found that bioethanol contributes to accelerating the material ageing.
Later work by Mielczarek et al. (2017) proposed a detailed reaction mechanism of toluene autoxidation. RMG (Gao et al., 2016) was employed to generate the reaction mechanism, which consists of 2309 reactions among 173 species. The thermodynamic data of 32 key species were updated using a quantum chemistry calculation which qualitatively improved the model compared to the original version from RMG. The PetroOXY method was used to validate this model, which involved experimental data from three different temperatures. The model was not able to predict the induction period of the PetroOXY test, and a sensitivity analysis discovered that the rate parameters had little impact on the temperature.
The approach for developing autoxidation mechanisms of jet fuels relies on the measurement of oxygen depletion rate and oxidation products. Jones and Balster (1993) identified the formation of insolubles in jet fuel oxidation using a near-isothermal flowing test rig. The experiment measured the oxygen depletion during the thermal stress and insolubles that were captured in the filters. The study explained that the oxygen consumption yields peroxy radicals (ROO) formation, which is the precursor of insoluble particles. A more detailed oxidation mechanism of various functional group has been reported by Denisov and Afanas'ev (2005). Later approach of autoxidation study incorporated more species detection, such as gas chromatography, to provide wider validation of the model (Kuprowicz et al., 2007; Sander et al., 2015). To the author's best knowledge, the most recent study of jet fuel autoxidation is reported by Alborzi et al. (2021) who optimised and validated the kinetic model of paraffinic solvent for the aviation fuel model that is proposed previously by the Zabarnick group.
This paper evaluates the oxidation stability of ethanol/kerosene blend with various concentrations as well as the strategy to improve the oxidation stability through an evaluation of antioxidant addition. A similar investigation was performed by using surrogate mixtures to evaluate the accuracy of the surrogate in representing the aviation kerosene. A python code that employs RMG and published mechanisms are proposed to model the pressure drop experimental data.
2. Experiment materials and method
The aviation kerosene sample was a real jet A-1 from Shell, while n-decane and n-dodecane sample were obtained from Sigma-Aldrich with ≥ 99% purity. The ethanol sample was purchased from Fisher with 99.8% purity, while 1,2,4 trimethylbenzene sample was acquired from Fisher with 99.8% purity. Methylcyclohexane and o-xylene samples were purchased from Alfa Aesar with 99% purity. Nine antioxidants that were used in this study are butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), tert-butylhydroquinone (TBHQ), propyl gallate (PG), pyrogallol (PY), 2,6-di-tert-butylphenol (DTBP), decalin, tetralin, 2-tert-butyl-4,6-dimethylphenol (TBMP). Eight antioxidants were purchased from Sigma-Aldrich, while TBMP was obtained from ChemCruz. Most of the antioxidants have 99% purity or higher except TBHQ and TBMP, which have 97 and 98% purity, respectively.
For each PetroOXY test, a 5 mL sample is required, and this was prepared by using a Costar sterile pipette and a pipette filler with an accuracy of 0.1 mL. For blending and handling the fuel, the sample was introduced in a sample bottle and mixed for approximately one minute to ensure the uniformity of the sample. An accurate scale, similar to the scale that was used for the MFC calibration, which was the TB-125D from Denver Instrument Germany, with an accuracy of 0.1 mg, was used to prepare the antioxidant samples. Approximately 5 mg of the antioxidant samples were introduced to the 5 mL ethanol sample, which results in 1 g/L antioxidant concentration.
The PetroOXY was obtained from Anton Paar [20], and it was connected to the Oxy logger software for data acquisition. A cleaning process was performed prior to each test to ensure the purity of the testing chamber. When a test was completed, the fuel sample was removed from the testing chamber by using a pipette. Then, a purging procedure was performed to clean any contaminant at the inlet and the outlet of the oxygen supply. In order to clean the surface of the gold dish and the lid, a special soft sheet and acetone were used to ensure that the dish surface was not damaged and oil was completely removed from the surface. An O-ring was used to seal the testing chamber from leaking, and this ring was replaced after a test run.
During the test, the PetroOXY device was set to the ASTM D7545 standard temperature and pressure, which are 700 kPa and 140 ∘C, respectively. The ASTM D7545 regulates the standard test method for Oxidation Stability of Middle Distillation Fuels, and this method has been used in the previous studies (Mielczarek, 2015; Zhou et al., 2017; Mielczarek et al., 2017; Alborzi et al., 2021). Once the fuel sample was placed, the testing chamber was pressurised with oxygen to 700 kPa. After it reached the targeted pressure, the pressurised gas in the sampling chamber was purged and then re-pressurised to 700 kPa. This procedure was performed to minimise nitrogen contamination from the air. The oxygen gas was supplied by BOC with 99.999% purity (N5) and connected using an oxygen-clean tubing. Subsequently, the testing chamber was heated to 140 ∘C, which caused the increase in the pressure inside the testing chamber. Afterwards, the temperature was maintained, and the data of the reactor pressure, temperature, and heating power was recorded by the PetroOXY device. The pressure inside the testing chamber decreased with time due to the consumption of oxygen by the fuel, which reduced the amount of oxygen gas at the headspace of the testing chamber. After a 10 per cent in pressure drop was achieved from the maximum pressure, the test was finished, and the data can be downloaded from the data logging software. The time required to achieve the 10 per cent pressure drop is also called the induction period.
The repeatability of the PetroOXY device has been excellent in the test results. A verification fluid, which was obtained from the manufacturer, was used to check the repeatability of the PetroOXY device. The result showed that the time to achieve a 10 per cent pressure drop, which was indicated by the PetroOXY device, was in the range of the verification fluid certificate. The 10 per cent pressure drop for the fluid was certified by Anton Paar at 93 minutes with 9 minutes deviation for 700 kPa and 140 ∘C, while the test achieved it for 99.55 minutes. Moreover, the repeatability test was performed twice by measuring the pressure drop of 50 per cent ethanol/jet A-1 mixture. The results showed that the time to achieve a 10 per cent pressure drop was shifted by 0.8 minutes from the first test while the maximum pressure shifted by 2 kPa. Therefore, for this study, the samples were only tested once due to the reliability of the PetroOXY test. Fig. 1 illustrates the research methodology that is used in this study.
Figure 1.
Schematic of the research methodology of this study.
3. Modelling approach
The PetroOXY experiments are set up so that a liquid fuel sample is heated and pressurised in pure O2. As the O2 reacts with the liquid sample, the pressure decreases. The rate of reaction with O2 is then characterised by the length of time it takes for the pressure to drop by a specified percentage, allowing the comparison of thermal stability between different fuels.
In order to model this experimental approach, it is necessary to calculate how much O2 dissolves into the liquid phase and is therefore available to react. As the dissolved O2 reacts, it is replaced by O2 from the gas phase, which reduces the pressure. Everything is modelled assuming no spatial variations in concentration, and reaction progress is monitored across time.
Typical perfectly stirred reactor (PSR) calculations solve the following ordinary differential equations (ODEs) for species (Glarborg et al., 1986):
| (1) |
where is the mass fraction for species k, is the net molar production rate for species k from reactions, is the molecular weight of species k and ρ is the total density, usually calculated from the equation of state (EoS) model, which will often be the ideal gas model. For the liquid phase kinetics in this work, the density is assumed to be constant, and the ODE solver is instead used to integrate the simpler equation for the molar concentration:
| (2) |
The concentration of O2 in the liquid phase is assumed to obey Henry's law at all times, such that
| (3) |
where P is the gas pressure and kH is the Henry's constant. Using the ideal gas law, this can be represented in terms of the number of moles of O2 in the headspace as
| (4) |
where is the number of moles of O2 in the headspace, R is the universal gas constant, T is the gas temperature, which is kept constant throughout, and is the volume of the headspace.
It is assumed that any consumption of O2 in the liquid phase is instantaneously replenished by Henry's law, so any chemical consumption of O2 is directly taken from the gas-phase, so the consumption of can be described by
| (5) |
where is the volume of the liquid sample. The first term on the right hand side of Eq (5) represents any chemical consumption of O2 and the second term represents any O2 absorbed (or released) from the liquid phase. By differentiating Eq. (3) and substituting it into Eq. (5) produces
| (6) |
Rearranging Eq. (6) gives
| (7) |
The set of ODEs to solve are now Eq. (2) for each species and Eq. (7) for the number of moles of O2 in the headspace (and thus the pressure of the system).
Finally, the total volume, , of the PetroOXY is kept constant at 27.7 ml. Nominally, the fuel sample is measured to be 5 ml. The thermal expansion of the fuel sample is taken into account based on the density of the fuel at standard conditions and at elevated temperature, usually at 413 K, and the volume of the headspace is calculated as .
The implementation is based on the custom ODE example from the Cantera (Goodwin et al., 2018) installation. Cantera is used for the basic thermodynamic and kinetic calculations, and the underlying system of equations is solved using the SciPy integrator. The solution is provided in the form of two python scripts, one containing the base reactor class and the other containing the case information for each run.
The base PetroOxyReactor class contains all the code to call up the SciPy integrator and to initialise and write out results. The PetroOxyODE class is used to return the required for the ODE solver, Eqs. (2) and (7). The file to run the reactor contains all the information to run a single case. Ideally, all runs should be made with the same PetroOxyReactor class but with different settings to maintain consistency in the calculations.
4. Results
4.1. Ethanol/jet A-1 blend
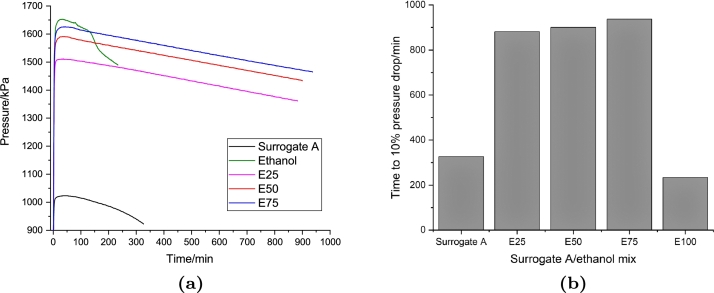
Fig. 2 shows the results of the PetroOXY test for the jet A-1/ethanol mixtures at 700 kPa and 140 ∘C. It is clearly seen that the pure jet A-1 fuel has the highest oxidation stability by achieving 10 per cent pressure drop in approximately 24 hours. Meanwhile, the ethanol has lower oxidation stability than jet A-1 and required less than six hours to reach 10 per cent pressure drop. Consequently, increasing the ethanol concentration in the jet A-1 blend decreased the stability of the fuel. All samples showed a different pressure rise with the increase in temperature. Jet A-1 showed the lowest maximum pressure, which is approximately 1000 kPa, while ethanol had the highest maximum pressure during the test, which is approximately 1650 kPa. The blends indicate that the peak pressure escalates with the increase of ethanol concentration in the blend. The higher maximum pressure in the ethanol sample might be caused by the higher volatility of ethanol than the jet A-1, where the vapour pressure of ethanol is more than 15 times that of kerosene at room temperature (The Engineering Tool Box, Accessed:30 January 2020).
Figure 2.
(a) Pressure drop time history and (b) required time to 10 per cent pressure drop of the PetroOXY test of the jet A-1/ethanol fuel blends.
The longer induction period in the jet A-1 sample can be caused by several factors, such as the presence of the antioxidant, the chemical characteristic, and the oxygen content in the sample. Unlike the ethanol sample that was obtained from a pure solvent, the jet A-1 sample was taken from commercial jet fuel products. Consequently, antioxidant was added to the jet fuel product up to 24 mg/L as stated in the jet fuel standards and the presence of antioxidant can improve the oxidation stability of the fuel (Chevron, 2007). Also, a natural antioxidant, such as organic sulphur compounds, may exist in the jet A-1 sample, which may have a sulphur content of up to 0.3 per cent. It was reported by Bolshakov (1987) that the sulphur compounds inhibit the oxidation of hydrocarbon mixtures.
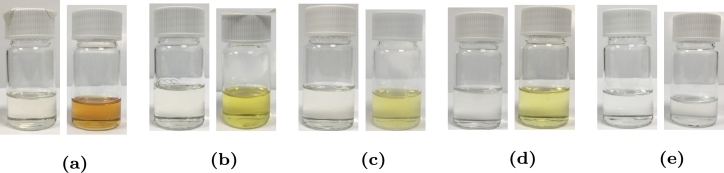
According to the study by Naegeli (1999), the sulphur content in the jet fuel causes the formation of gums and deposits. Also, the fuel sample that requires a higher temperature and a longer induction period generate more gums and deposits during the autoxidation process. A recent study by Rawson et al. (2018) reported that the addition of elemental sulfur decreases the oxygen depletion rate and yields a large mass of deposit. Fig. 3 shows the visual appearance of the fuel samples before and after the tests. It is observed that the change of colour was dominant in the jet A-1 sample, while by increasing the ethanol concentration, the colour of the sample after the test becomes even more clearer. The increase of oxygen concentration in the liquid may also contribute to the acceleration of the autoxidation process. Referring to Henry's coefficient data from Denisov and Afanas'ev (2005), the coefficient for ethanol is approximately three times greater than that of kerosene, which leads to approximately three times higher oxygen concentration in the fuel at the same partial pressure of oxygen. Moreover, the maximum pressure during the test of the ethanol sample was higher than the jet A-1 sample, and this may accelerate the autoxidation process due to the increase in the oxygen concentration.
Figure 3.
The visual appearance of the jet A-1/ethanol blend samples (left) before and (right) after the PetroOXY test for (a) pure jet A-1, (b) E25, (c) E50, (d) E75, and (e) pure ethanol.
4.2. Multi component surrogate
Unfortunately, there was no analytical equipment that can be used to detect the contributing species in the autoxidation process. Alternatively, modelling work was attempted to explain the autoxidation process of the samples. Since it was difficult to model the real jet A-1 due to the numerous compounds that exist in the real fuel, two surrogate models were tested to replicate the behaviour of the real jet A-1 fuel. Surrogate A consists of 80:20 n-decane and 1,2,4 trimethyl benzene by mass, while Surrogate B comprises 60:20:20 n-dodecane, o-xylene, and methylcyclohexane by volume. These surrogates were similar to the study of surrogate flames in the previous chapter, which was based on the study from Honnet et al. (2009) and Humer et al. (2007).
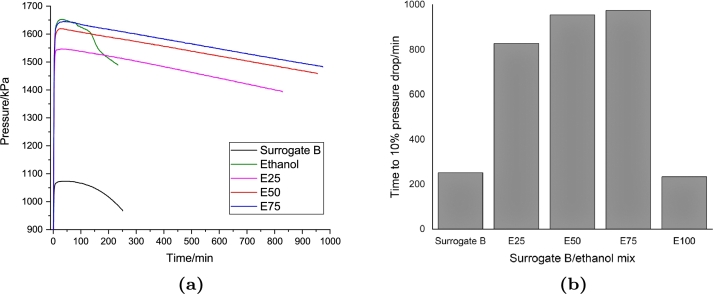
Figure 4, Figure 5 present the results of the PetroOXY test of the surrogate A and B, respectively. The induction time of both surrogates was much less than the real jet A-1. This might be caused by the absence of additives and a natural antioxidant in the sample. The effect of the ethanol addition to the surrogate was also different than the jet A-1. The induction time of both surrogates increased with the increase of ethanol concentration. Meanwhile, pure ethanol has a slightly lower induction time than both surrogates. The behaviour of the pressure history during the test was similar to the jet A-1 samples, where the maximum temperature increased with increasing ethanol concentration. Meanwhile, the maximum pressure of the surrogates was similar to that of the jet A-1 sample.
Figure 4.
(a) Pressure drop time history and (b) required time to 10 per cent pressure drop of the PetroOXY test of the surrogate A/ethanol fuel blends.
Figure 5.
(a) Pressure drop time history and (b) required time to 10 per cent pressure drop of the PetroOXY test of the surrogate B/ethanol fuel blends.
4.3. Single component surrogate
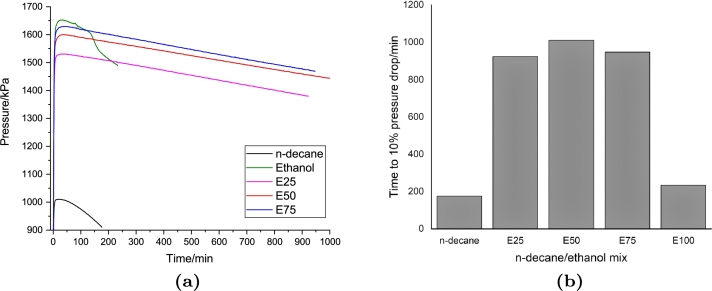
A modelling work of the multi-component surrogates was attempted using a reaction mechanism generator. However, the multi-component surrogates required more computational resources and time. Alternatively, a single component surrogate was used for the modelling work. Figure 6, Figure 7 show the test results of n-decane and n-dodecane sample, respectively. A different effect of ethanol addition to both the n-decane and n-dodecane samples was shown in the 75 per cent ethanol concentration, where the induction time decreased from the 50 per cent case. Meanwhile, in both multi-component surrogates, the induction time at 75 per cent ethanol blend was higher than the 50 per cent case.
Figure 6.
(a) Pressure drop time history and (b) required time to 10 per cent pressure drop of the PetroOXY test of the n-decane/ethanol fuel blends.
Figure 7.
(a) Pressure drop time history and (b) required time to 10 per cent pressure drop of the PetroOXY test of the n-dodecane/ethanol fuel blends.
The maximum pressure during the test was not very different between the real jet A-1, multi, and single component surrogates. The maximum pressure during the test increased to approximately a similar value with a similar increase in the ethanol concentration. This indicates that the maximum pressure during the test was controlled by the volatility of ethanol. By removing the cyclic compound in the single surrogate component, the stability of the 25 and 50 per cent ethanol mixture slightly increased. Meanwhile, the stability of n-decane was slightly higher than the n-dodecane. This might be related to the finding in the study of biodiesel, where the longer molecule chain and a less saturation in the C-H bond caused the reduction of the fuel oxidation stability (Saluja et al., 2016). In single and multi-component surrogates, the presence of ethanol increases the oxidation stability, while pure ethanol has a slightly higher induction time than the single component surrogates and a lower induction time than the multi-component surrogates.
4.4. Antioxidant test
Despite the ethanol addition improving the oxidation stability of the jet fuel surrogates, it behaves contrary to the real jet A-1 fuel. In order to enhance the oxidation stability of ethanol, several antioxidants were tested to determine their effect on the induction time of ethanol during the PetroOXY test. The selected antioxidants were studied in several published studies to improve the fuel oxidation stability, 1 g/L of the antioxidants were added to the ethanol sample.
Fig. 8 presents the time history and the induction time of the ethanol sample with 1 g/L antioxidant addition from the PetroOXY test. From these results, the addition of the antioxidants to the ethanol sample increases the induction time while each antioxidant showed different improvement to the ethanol oxidation stability. A slight extension to the induction time was found in the propyl gallate addition, while the other antioxidants enhance the induction time by more than three times. The best improvement in ethanol stability was found in the pyrogallol addition. The addition of these antioxidants did not affect the maximum pressure during the test, which reached 1650 kPa and then dropped in a different manner for each antioxidant.
Figure 8.
(a) Pressure drop time history and (b) required time to 10 per cent pressure drop of the PetroOXY test of the 1 g/L addition of antioxidants to ethanol.
The selected antioxidants, except decalin and tetralin, are monohydroxy or polyhydroxy phenolic antioxidants. Generally, the antioxidants delay the autoxidation process by preventing the formation of the intermediate peroxyl radical (Zhou et al., 2016). The antioxidants donate the hydrogen atom to the radical and produce less reactive species. The radical antioxidant species are relatively stable due to the delocalisation of radical electron, and furthermore, it can react with other radicals to form a stable species.
The use of PY, PG, TBHQ, BHA and BHT for improving the oxidation stability of biodiesel has been studied by Zhou et al. (2016). The study shows that the stability of the biodiesel sample increased with the increase in the antioxidant concentration, while the increase in temperature decreases the induction time. A similar finding with this study was found for the performance of the PY, which was the best in increasing the oxidation stability of the fuel compared to the other antioxidants. Meanwhile, the results of the other four antioxidants were different in this study, where the PG showed the least effective antioxidant while in the study of biodiesel, it was slightly less effective than the PY. The performance of decalin and tetralin as an antioxidant for HEFA-SPK has been studied by Amara et al. (2016), which shows the inhibition behaviour to the autoxidation process at low concentration. The DTBP and TBMP are commonly used in jet A-1 to inhibit the peroxidation of hydrocarbons which leads to deposit formation by autoxidation (Bernabei et al., 2000). Therefore, while they are effective for increasing the ethanol stability, they are also compatible with jet A-1.
4.5. Modelling the PetroOXY test
A modelling work was performed to model the autoxidation during the PetroOXY test. There are three samples that were modelled, which are ethanol, n-decane, and n-dodecane. The reaction mechanisms of these samples were generated by employing the Reaction Mechanism Generator (RMG) (Gao et al., 2016). The reason for using the RMG is the availability of the liquid phase reactor, which represent the PetroOXY reactor. The version of the RMG and the database was 2.3.0, which was executed in the university high-performance computer facility (ShARC). The RMG requires an input file to specify the initial reactant species, its concentration, reactor temperature, and the duration of the reaction. A similar condition to the real PetroOXY test was given for the temperature and the fuel concentration. Meanwhile, the concentration of oxygen in the liquid phase was determined by using Henry's law and the maximum pressure during the PetroOXY test. The concentration of oxygen was set to a constant value as recommended by the documentation of the RMG for more optimum reaction mechanism generation.
The output of the RMG run is the reaction mechanism in CHEMKIN and Cantera format as well as the simulated mole fraction of the contributing species. For the ethanol case, the generated reaction mechanism has 13 species and 26 reactions. Meanwhile, the n-decane mechanism has 11169 reactions among 193 species, and the n-dodecane mechanism has 262 species and 23083 reactions. The number of species and reactions increase with the larger molecule, and this consumed much of the computational resource to run these mechanisms. Since the RMG liquid reactor was not able to calculate the pressure in the headspace of the PetroOXY testing chamber, a python code was employed to calculate this parameter.
Fig. 9 presents the simulated pressure profile obtained from the model for ethanol, n-decane, and n-dodecane, as well as a comparison with the experimental data. It can be seen that the model ignores the pressure increase due to the temperature rise in the testing chamber. It starts from the maximum pressure and then drops with the oxygen consumption. The prediction of the pressure drop using the RMG mechanism was most accurate for the ethanol case, while the prediction for the n-decane and n-dodecane show a faster pressure drop than the experimental data.
Figure 9.
Simulation of the PetroOXY tests of the (a) ethanol, (b) n-decane, and (c) n-dodecane samples and the comparison with the experimental data.
The simulated mole fraction of the fuels and oxygen are similar in all cases where it decreases with time. The consumption of fuels and oxygen is caused by the initiation reactions which produces intermediates. Mostly, n-dodecane, n-decane, and ethanol undergo an H-abstraction reaction with oxygen to form dodecyl, decyl, and ethoxy radicals, respectively. Furthermore, the abstracted hydrogen atom and oxygen molecule produce the hydroperoxyl radical. The profile of the mole fraction of these intermediates radicals is relatively similar, which increases and peaks with the consumption of the fuel and then decreases rapidly with different rates. An exception to this trend was found in the concentration profile of hydroperoxyl in the ethanol case, where it increases continuously.
The intermediates that are formed during the initiation process react with more stable species and forms more radicals during the propagation step. Finally, these radicals form stable species, which is the termination process of the autoxidation process. In the autoxidation of ethanol, the model indicates that there are three main products of autoxidation which are hydrogen peroxide (H2O2), acetaldehyde (C2H4O), and hydroperoxy ethanol (C2H6O3). For the case of the n-decane and n-dodecane, the RMG reaction path visualiser could not generate the reaction path analysis. This may be caused by the size of the reaction mechanism that is much larger than the ethanol mechanism. Alternatively, the reaction path analyser from the CHEMKIN closed-homogenous reactor was used.
For the n-decane and n-dodecane, relatively similar functional group reactions were found following the formation of the alkyl radical (R•). The alkyl radical reacts with oxygen to form the peroxyl radical (ROO) or with hydroperoxyl (HO2) to form the hydroperoxide (ROOH). Furthermore, the hydroperoxide can undergo more H-abstraction in the other carbon atom to form more ROOH function through the same mechanism and forms HOOR1-R2OOH. The ROOH formation is the termination step of the n-decane and n-dodecane autoxidation, thus the concentration of ROOH increases with the consumption of oxygen. Meanwhile, the concentration of ROO peaks then reduces due to the formation of ROOH. The abundant species in the n-decane and n-dodecane mechanisms are caused by the numerous possibility of ROOH isomers of decyl and dodecyl radical.
4.6. Mechanism optimisation
An attempt to improve the accuracy of the n-decane and n-dodecane mechanisms for predicting the experimental data was performed by tuning the rate parameters of the sensitive reactions to the oxygen consumption. The sensitivity analysis was performed by using the RMG reactor feature as presented in Figs. 10a, 10b, and 10c. It was found that the most sensitive reactions to the oxygen consumption were the reactions involving dodecyl or decyl radicals and oxygen in the n-docedane or n-decane cases, respectively. By modifying the reaction rate parameters of these reactions, the prediction of the pressure drop becomes closer to the experimental data as illustrated in Figs. 9b and 9c. Table 1 shows the comparison of the modified and original rate parameters that were generated by RMG. However, this adjustment in the rate parameter may not be accurate since the rate parameters of the reactions were changed by up to seven orders of magnitude. This may contradict the rate parameter from previous studies.
Figure 10.
Oxygen sensitivity analysis of the RMG simulations of (a) ethanol, (b) n-decane, and (c) n-dodecane at the PetroOXY test condition.
Table 1.
The modified recombination reactions (R• + O2 = ROO) in (top) n-decane and (bottom) n-dodecane mechanisms from RMG.
| Modified reactions |
A/(cm3⋅mole−1⋅s−1) |
|
|---|---|---|
| original | modified | |
| C10H21(16) + oxygen(2) = S(50) | 7.54E+12 | 7.54E+6 |
| C10H21(11) + oxygen(2) = S(102) | 7.54E+12 | 7.54E+8 |
| C10H21(13) + oxygen(2) = S(78) | 7.54E+12 | 7.54E+6 |
| C12H25(19) + oxygen(2) = S(57) | 7.54E+12 | 7.54E+7 |
| C12H25(11) + oxygen(2) = S(144) | 7.54E+12 | 7.54E+6 |
| C12H25(14) + oxygen(2) = S(117) | 7.54E+12 | 7.54E+5 |
Many studies have evaluated the rate parameters of R• + O2 → ROO in the autoxidation reaction mechanism of jet fuels and surrogates. Kuprowicz et al. (2007) proposed a reaction mechanism for predicting the autoxidation of jet fuel which was refined by the species detection of the oxidised jet fuel samples. In this study, the proposed value of the A-factor of peroxide species formation of cm3⋅mole−1⋅s−1. This value was also used by Liu et al. (2019) for modelling the deposition formation in the thermal oxidation of aviation kerosene.
Ben Amara et al. (2013) studied n-dodecane autoxidation in a Rancimat experiment and proposed a reaction mechanism that was validated with the induction period data from the experiment. This study proposed the value of the A-factor as cm3⋅mole−1⋅s−1, which is similar to the original RMG mechanism in Table 1. Also, the reaction mechanism from Ben Amara et al. (2013) was employed in the python code for modelling the pressure drop data of n-dodecane from the current work. The results show that by using the model from Amara et al., the pressure drop prediction becomes closer to the current experimental data as illustrated in Fig. 9c, but it still underpredicts the experimental pressure drop data.
Considering these findings, the modified value of the rate parameters of the peroxide formation reaction is much beyond the recommended value from the literature. Thus, a more comprehensive method of optimisation of the RMG mechanism is required to improve the accuracy of the pressure drop prediction.
5. Conclusion
For the utilisation of ethanol as a drop-in blend for jet A-1, the stability of the fuel needs to be evaluated, and there is no published research that studies the autoxidation of these fuel blends. Thus, this work reports a novel study on the oxidation stability of the ethanol/jet A-1 blend by employing the PetroOXY method with different ethanol concentrations. The findings of this study are as follows:
-
•
The results show that with the increase in the ethanol concentration, the stability of the fuel becomes lower, while the maximum pressure during the test increases with the increase in the ethanol concentration. A visual assessment of the sample after the test is presented, where a cleaner sample is obtained with a higher ethanol concentration.
-
•
In addition, this work has extended the autoxidation study of fuels to the multi and single component surrogates to provide an experimental validation to the development of the kinetic model. The effect of ethanol addition to the multi-component surrogates improves the stability of the mixture, while the maximum stability was found at 50% for a single-component surrogate.
-
•
An accurate kinetic modelling of fuel autoxidation has been developed, and this work evaluated the modelling tools that are available in the literature for modelling the PetroOXY tests. Reaction mechanisms for n-decane, n-dodecane, and ethanol from RMG have been employed for the modelling PetroOXY by using a custom code. The model shows good accuracy for the ethanol case, while a faster pressure drop than the experimental data was found for the n-decane and n-dodecane. An optimisation of the reaction rate parameters has been demonstrated to improve the accuracy of the reaction mechanism in predicting the pressure drop validation.
-
•
An antioxidant addition is one of the jet fuel additives that improve the fuel stability, but there is no study on the antioxidant addition to ethanol stability. Nine antioxidants have been tested to improve the stability of ethanol at 1 g/L. The result shows that the effectiveness of the antioxidants is following this order: PY > Decalin > DTBP > Tetralin > BHT > MTBP > BHA > TBHQ > PG. This should be taken into account when selecting an effective antioxidant for bioethanol.
Declarations
Author contribution statement
A.S. Auzani: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A.G. Clements: Contributed reagents, materials, analysis tools or data.
K.J. Hughes: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
D.B. Ingham: Performed the experiments; Wrote the paper.
M. Pourkashanian: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by the Indonesia Endowment Fund for Education (LPDP).
Data availability statement
Data included in article/supp.material/referenced in article
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors gratefully acknowledge Dr Simon Blakey and Dr Ehsan Alborzi for the fruitful discussions on aviation fuel oxidation stability.
References
- Alborzi E., Dwyer M.R., Parks C.M., Sheikhansari A., Mielczarek D.C., Zanganeh M., Meijer A.J.H.M., Blakey S.G., Pourkashanian M. Construction of a reduced chemical kinetic mechanism for autoxidation of n-paraffinic solvent – a model for aviation fuel. Fuel. 2021;294 [Google Scholar]
- Amara A.B., Kaoubi S., Starck L. Toward an optimal formulation of alternative jet fuels: enhanced oxidation and thermal stability by the addition of cyclic molecules. Fuel. 2016;173:98–105. [Google Scholar]
- Arun J., Gopinath K.P., SundarRajan P., Malolan R., AjaySrinivaasan P. Hydrothermal liquefaction and pyrolysis of amphiroa fragilissima biomass: comparative study on oxygen content and storage stability parameters of bio-oil. Bioresource Technology Reports. 2020;11 [Google Scholar]
- Batts B.D., Fathoni A.Z. A literature review on fuel stability studies with particular emphasis on diesel oil. Energy Fuels. 1991;5:2–21. [Google Scholar]
- Ben Amara A., Nicolle A., Alves-Fortunato M., Jeuland N. Toward predictive modeling of petroleum and biobased fuel stability: kinetics of methyl oleate/n-dodecane autoxidation. Energy Fuels. 2013;27:6125–6133. [Google Scholar]
- Bernabei M., Bocchinfuso G., Carrozzo P., De Angelis C. Determination of phenolic antioxidants in aviation jet fuel. J. Chromatogr. A. 2000;871:235–241. doi: 10.1016/s0021-9673(99)01274-1. [DOI] [PubMed] [Google Scholar]
- Bolshakov G. The effect of organic sulfur compounds on oxidation processes of hydrocarbon fuels. Sulfur Rep. 1987;7:379–392. [Google Scholar]
- Braz D.S., Mariano A.P. Jet fuel production in eucalyptus pulp Mills: economics and carbon footprint of ethanol vs. butanol pathway. Bioresour. Technol. 2018;268:9–19. doi: 10.1016/j.biortech.2018.07.102. [DOI] [PubMed] [Google Scholar]
- Buffi M., Valera-Medina A., Marsh R., Pugh D., Giles A., Runyon J., Chiaramonti D. Emissions characterization tests for hydrotreated renewable jet fuel from used cooking oil and its blends. Appl. Energy. 2017;201:84–93. [Google Scholar]
- Chansauria P., Mandloi R.K. vol. 5. 2018. Effects of Ethanol Blends on Performance of Spark Ignition Engine-A Review; pp. 4066–4077. (Materials Today: Proceedings). [Google Scholar]
- Chevron 2007. https://www.chevron.com/-/media/chevron/operations/documents/aviation-tech-review.pdf Aviation Fuels Technical Review. Technical Report. USA.
- de Jesus Silva A.J., Contreras M.M., Nascimento C.R., da Costa M.F. Kinetics of thermal degradation and lifetime study of poly(vinylidene fluoride). (PVDF) subjected to bioethanol fuel accelerated aging. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisov E.T., Afanas'ev I.B. CRC Press; 2005. Oxidation and Antioxidants in Organic Chemistry and Biology. [Google Scholar]
- Dhande D.Y., Sinaga N., Dahe K.B. Study on combustion, performance and exhaust emissions of bioethanol-gasoline blended spark ignition engine. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C.W., Allen J.W., Green W.H., West R.H. Reaction mechanism generator: automatic construction of chemical kinetic mechanisms. Comput. Phys. Commun. 2016;203:212–225. [Google Scholar]
- Glarborg P., Kee R.J., Grcar J.F., Miller J.A. 1986. PSR: A FORTRAN Program for Modeling Well-Stirred Reactors. Sandia Report SAND86-8209. [Google Scholar]
- Goodwin D.G., Speth R.L., Moffat H.K., Weber B.W. Cantera: an object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes. 2018. version 2.4.0.
- Honnet S., Seshadri K., Niemann U., Peters N. A surrogate fuel for kerosene. Proc. Combust. Inst. 2009;32:485–492. [Google Scholar]
- Humer S., Frassoldati A., Granata S., Faravelli T., Ranzi E., Seiser R., Seshadri K. Experimental and kinetic modeling study of combustion of jp-8, its surrogates and reference components in laminar nonpremixed flows. Proc. Combust. Inst. 2007;31:393–400. [Google Scholar]
- Jones E.G., Balster W.J. Phenomenological study of the formation of insolubles in a jet-a fuel. Energy Fuels. 1993;7:968–977. [Google Scholar]
- Ju D., Sun X., Jia X., Huang Z., Qiao X., Han D., Huang Z. Experimental investigation of the atomization behavior of ethanol and kerosene in acoustic fields. Fuel. 2017;202:613–619. [Google Scholar]
- Karavalakis G., Stournas S., Karonis D. Evaluation of the oxidation stability of diesel/biodiesel blends. Fuel. 2010;89:2483–2489. [Google Scholar]
- Kuprowicz N.J., Zabarnick S., West Z.J., Ervin J.S. Use of measured species class concentrations with chemical kinetic modeling for the prediction of autoxidation and deposition of jet fuels. Energy Fuels. 2007;21:530–544. [Google Scholar]
- Liu Z., Tang S., Li Z., Qin Z., Yuan S., Wang L., Wang L., Zhang X., Liu G. An improved kinetic model for deposition by thermal oxidation of aviation hydrocarbon fuels. Fuel. 2019;258 [Google Scholar]
- Malani R.S., Batghare A.H., Bhasarkar J.B., Moholkar V.S. Kinetic modeling and process engineering aspects of biodesulfurization of liquid fuels: review and analysis. Bioresour. Tech. Rep. 2021;14 [Google Scholar]
- Mielczarek D.C. University of Leeds; 2015. Autoxidation behaviour of hydrocarbons in the context of conventional and alternative aviation fuels. Theses (PhD Theses) [Google Scholar]
- Mielczarek D.C., Matrat M., Amara A.B., Bouyou Y., Wund P., Starck L. Toward the accurate prediction of liquid phase oxidation of aromatics: a detailed kinetic mechanism for toluene autoxidation. Energy Fuels. 2017;31:12893–12913. [Google Scholar]
- Naegeli D.W. ASME 1999 International Gas Turbine and Aeroengine Congress and Exhibition. American Society of Mechanical Engineers Digital Collection; 1999. The role of sulfur in the thermal stability of jet fuel. [Google Scholar]
- Nikkhah A., Assad M.E.H., Rosentrater K.A., Ghnimi S., Van Haute S. Comparative review of three approaches to biofuel production from energy crops as feedstock in a developing country. Bioresour. Tech. Rep. 2020;10 [Google Scholar]
- Patra J., Ghose P., Datta A., Das M., Ganguly R., Sen S., Chatterjee S. Studies of combustion characteristics of kerosene ethanol blends in an axi-symmetric combustor. Fuel. 2015;144:205–213. [Google Scholar]
- Rao D.C.K., Syam S., Karmakar S., Joarder R. Experimental investigations on nucleation, bubble growth, and micro-explosion characteristics during the combustion of ethanol/jet a-1 fuel droplets. Exp. Therm. Fluid Sci. 2017;89:284–294. [Google Scholar]
- Rawson P.M., Webster R.L., Evans D., Abanteriba S. Contribution of sulfur compounds to deposit formation in jet fuels at 140 °C using a quartz crystal microbalance technique. Fuel. 2018;231:1–7. [Google Scholar]
- Ryu K. The characteristics of performance and exhaust emissions of a diesel engine using a biodiesel with antioxidants. Bioresour. Technol. 2010;101:S78–S82. doi: 10.1016/j.biortech.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Sadhukhan J., Martinez-Hernandez E., Amezcua-Allieri M.A., Aburto J. Economic and environmental impact evaluation of various biomass feedstock for bioethanol production and correlations to lignocellulosic composition. Bioresour. Tech. Rep. 2019;7 [Google Scholar]
- Saluja R.K., Kumar V., Sham R. Stability of biodiesel – a review. Renew. Sustain. Energy Rev. 2016;62:866–881. [Google Scholar]
- Sander Z.H., West Z.J., Ervin J.S., Zabarnick S. Experimental and modeling studies of heat transfer, fluid dynamics, and autoxidation chemistry in the jet fuel thermal oxidation tester (jftot) Energy Fuels. 2015;29:7036–7047. [Google Scholar]
- Shahir S.A., Masjuki H.H., Kalam M.A., Imran A., Ashraful A.M. Performance and emission assessment of diesel–biodiesel–ethanol/bioethanol blend as a fuel in diesel engines: a review. Renew. Sustain. Energy Rev. 2015;48:62–78. [Google Scholar]
- Song L., Liu T., Fu W., Lin Q. Experimental study on spray characteristics of ethanol-aviation kerosene blended fuel with a high-pressure common rail injection system. J. Energy Inst. 2016 [Google Scholar]
- The Engineering Tool Box Vapor and saturation pressure for some common liquids. Accessed:30 January 2020. https://www.engineeringtoolbox.com/vapor-pressure-d_312.html
- Zabarnick S. Chemical kinetic modeling of jet fuel autoxidation and antioxidant chemistry. Ind. Eng. Chem. Res. 1993;32:1012–1017. [Google Scholar]
- Zhou J., Xiong Y., Liu X. Evaluation of the oxidation stability of biodiesel stabilized with antioxidants using the rancimat and pdsc methods. Fuel. 2017;188:61–68. [Google Scholar]
- Zhou J., Xiong Y., Xu S. Evaluation of the oxidation stability of biodiesel stabilized with antioxidants using the petrooxy method. Fuel. 2016;184:808–814. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp.material/referenced in article