FIGURE 1.
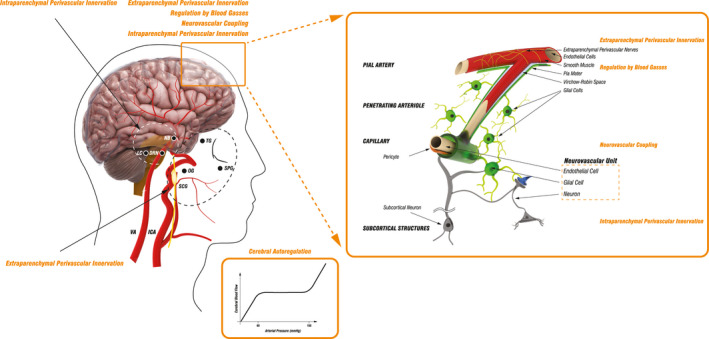
Schematic presentation of cerebrovascular tree structure and cerebral blood flow regulatory mechanisms. The brain receives arterial perfusion through two internal carotid arteries (ICA) and two vertebral arteries (VA) that interconnect intracranially through the circle of Willis, which gives rise to several vessels branching into progressively smaller arteries that run along the surface of the brain and then penetrate the brain tissue continuing deeper into the brain until they terminate in a network of capillaries. The main mechanisms regulating cerebral blood flow (CBF) include cerebral autoregulation (CA), activity of perivascular nerves, regulation by blood gasses, and neurovascular coupling. Cerebral autoregulation (CA) ensures CBF is maintained approximately constant across a wide range of mean arterial pressure (MAP), with neck vessels and large cerebral arteries serving as the first line of defense against hyperperfusion of the downstream microvasculature. CBF is highly dependent on extraparenchymal and intraparenchymal perivascular innervation. Extraparenchymal cerebral blood vessels are extensively innervated by autonomic and sensory nerve fibers deriving from the superior cervical ganglion (SCG), sphenopalatine ganglion (SPG), otic ganglion (OG), and the trigeminal ganglion (TG), while intraparenchymal vessels receive innervation through projections from subcortical neurons originating in locus coeruleus (LC), nucleus basalis (NB), and dorsal raphe nucleus (DRN). Pial arterioles are considered to be the main site of resistance regulation associated with changes in the partial pressure of blood gasses and of pH. Neurovascular coupling, which defines the association between neurons, microvascular endothelial cells, and glial cells, initiates local CBF changes on the level of capillaries. This signal is then transmitted upstream to remote vessels, including pial arterioles, which leads to broader blood flow changes. Additionally, neurovascular coupling may be modulated through subcortical neuronal activity. Internal carotid artery (ICA); vertebral artery (VA); superior cervical ganglion (SCG), otic ganglion (OT); sphenopalatine ganglion (SPG); trigeminal ganglion (TG); superior cervical ganglion (SCG); locus coeruleus (LC); dorsal raphe nucleus (DRN); nucleus basalis (NB)
