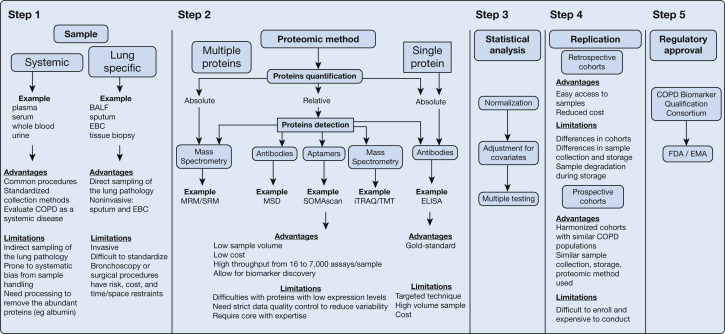
Figure 1.
Approach to judging a proteomic study. Brief description of the five steps to be considered when judging a well-designed proteomic study. Step 1: evaluation of samples used in the proteomic study with the advantages and limitations of systemic vs lung-specific samples. Step 2: evaluation of the proteomic method used in the study, including the platform (single vs multiplex), detection method (antibodies, aptamers, or mass spectrometry), and quantification (relative or absolute) with their advantages and limitations vs ELISA, the current criterion standard method. Step 3: evaluation of the statistical methods used with specific considerations for data quality control, normalization, and adjustment for covariates. Step 4: evaluation of the cohorts used for protein biomarker validation with advantages and limitations of retrospective vs prospective cohorts. Step 5: evaluation by the regulatory agencies involved in biomarker approval. BALF = BAL fluid; EBC = exhaled breath condensate; ELISA = enzyme-linked immunosorbent assay; EMA = European Medicines Agency; FDA = Food and Drug Administration; iTRAQ/TMT = isobaric tag for relative and absolute quantitation/tandem mass tags; MRM/SRM = multiple reaction monitoring/selective reaction monitoring; MSD = Meso Scale Discovery; SOMAscan = slow off-rate modified aptamers.