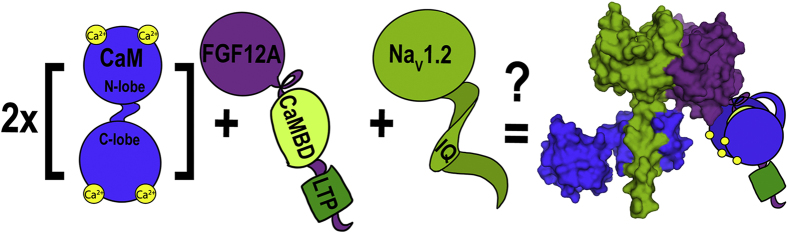
Figure 1.
The Shea group’s investigations revealed two novel CaM (blue) interaction domains on FGFs (purple; domains labeled CaMBD and LTP) with the CaMBD showing high affinity for calcium-saturated CaM. It is known that the NaV (green) IQ motif binds strongly to calcium-free (apo-)CaM. Together, these data suggest that upon CaM binding of calcium, the change in conformation results in CaM’s dissociation from the NaV channel to reassociate with the proximal FGF. Alternatively, a second CaM molecule may be present in the complex (6). CaM, calmodulin; CaMBD, CaM-binding domain; FGFs, fibroblast growth factors; LTP, long-term inactivation particle; NaVs, voltage-gated sodium channels.