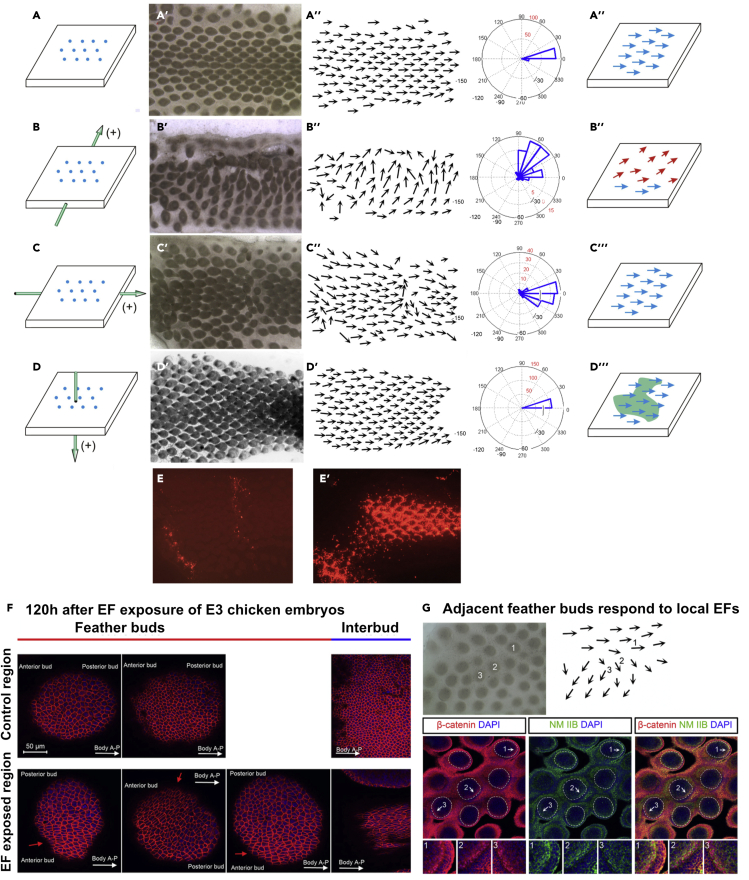
Figure 2.
Application of exogenous electric field on E7 skin explants in vitro alters feather bud orientations in a topological dependent fashion
(A–D) Schematic diagrams of EF flow. (A) Control skin explants with anterior (left) and posterior (right). (B) Exogenous EF exposure laterally through the skin explant with the anode (top) and cathode (bottom) poles positioned perpendicular to the A-P axis. (C) with the cathode and anode positioned along the A-P axis of the explant. (D) with the cathode above the epithelium and anode below the mesenchyme so the electrons flow from the top to the bottom of the explant perpendicularly. Feather buds (A′–D′) and their orientation (A″–D″) are shown for each condition (n = 7 skins). Gene transfer was assessed 24 hr after pulsed EF application of CMV-RFP with electrodes positioned as in panels B, C, and D.
(E) Few cells expressed the exogenous plasmid when electroporated along the A-P axis. (E′) Widespread RFP expression was seen when the EF was oriented along the epithelium-mesenchyme axis. (A‴–D‴) Schematic representation of the results.
(F) After transient EF exposure at E3, cell shape was assessed at E8 by staining the membranes with antibodies to E-cadherin. Cells in the EF exposed region (red arrows) within the anterior and posterior feather bud and the interbud became elongated along the axis of electroporation compared to nonelectroporated control regions. (F and G) Occasionally adjacent cells near the border of electroporated regions were subject to different EF field orientations inducing different polarity on each bud (G, top left panel). A schematic diagram shows electrode placement and EF polarity (G, top right panel). This can be seen more clearly in the polarity of beta-catenin, non-muscle myosin IIB (NM IIB) or both after immunostaining (G, bottom panels—see insets for enlargements of each bud). Size bars in panel F = 50 μM.