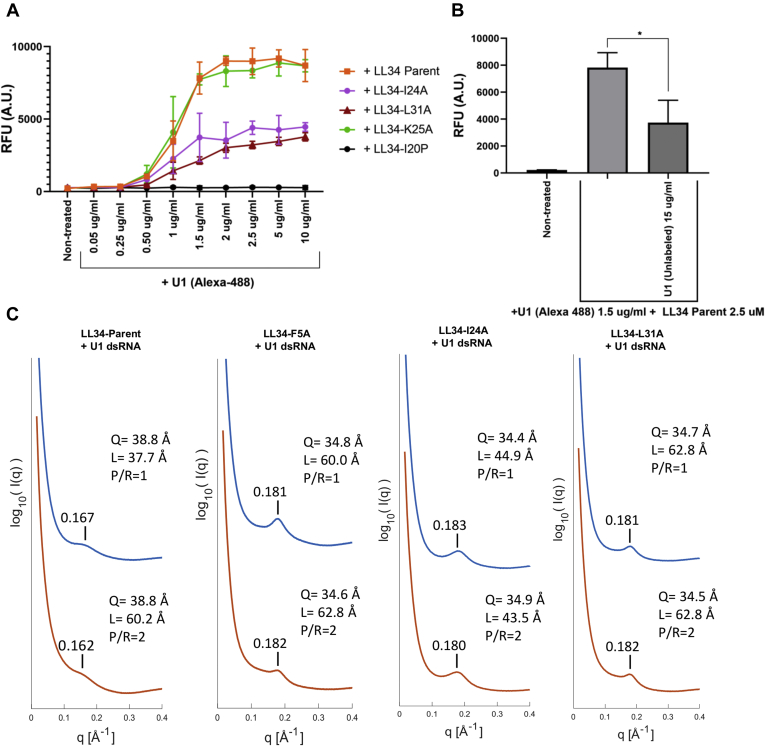
Figure 5.
Biophysical properties of binding of U1 dsRNA to LL-34 peptides and SRB1 protein.A, NHEKs were treated with Alexa 488–labeled U1 dsRNA or a combination of Alexa 488–labeled U1 dsRNA (0.05–10 μg/ml) and LL-34 peptides LL-34 parent, LL-34 I24A, LL-34 K25A, LL-34 L31A, and LL-34 I20P at 2.5 μM as shown. The cells were prechilled on ice for 15 min before the addition of U1 dsRNA and/or LL-34 peptides, and the surface binding of U1 dsRNA was allowed for 10 min. The amount of U1 dsRNA binding to NHEKs was assessed with a fluorometer, and data are represented as relative fluorescence units (RFUs) (n = 3). B, NHEKs were similarly treated with Alexa-labeled U1 dsRNA (1.5 μg/ml) and/or unlabeled U1 dsRNA (15 μg/ml) with the addition of LL-34 parent peptide at 2.5 μM. The experiment was performed on ice for 10 min, and the relative binding of Alexa 488 U1 dsRNA was assessed on a fluorometer and is shown as RFU (n = 3) (∗p < 0.05) one-way ANOVA. C, SAXS spectra of LL-34 peptides LL-34 parent, LL-34 F5A, LL-34 I24A, and LL-34 L31A incubated with U1 dsRNA. The peptide-U1 dsRNA (P/R) ratio is varied at 1 and 2 for all conditions. The U1 dsRNA interhelical spacing (Q) was calculated by the peak position. The domain size (L) was calculated by the full width at half maximum of the first peak. LL-34, 34-amino acid peptide; NHEKs, normal human epidermal keratinocytes; SAXS, small-angle X-ray scattering.