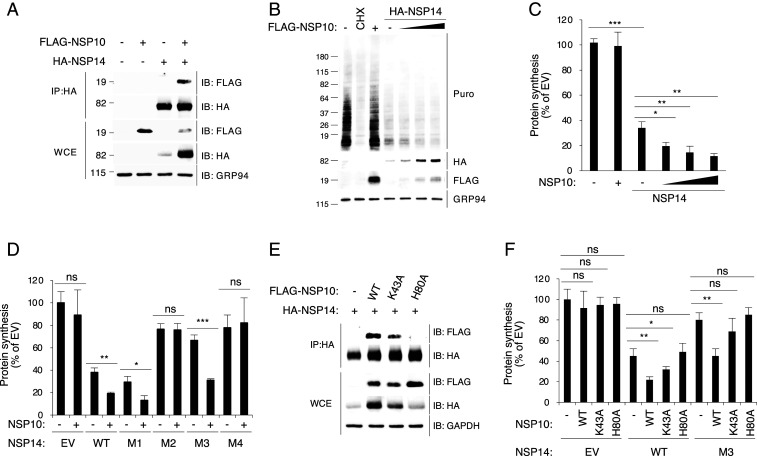
Fig. 5.
NSP14−NSP10 complex formation enhances the translation inhibition activity of NSP14. (A) The 293T cells were transfected with plasmids encoding indicated proteins for 24 h. Cell lysates were subjected to IP using mouse anti-HA antibody and followed by immunoblotting with rabbit anti-HA and anti-FLAG antibodies. WCE, whole cell extract. (B) The 293T cells were transfected with plasmids encoding NSP10 or NSP14 for 24 h and puromycin labeled for 15 min. Puromycin incorporation was determined by immunoblotting using anti-puromycin antibody (Puro). HA-tagged NSP14 and FLAG-tagged NSP10 proteins were detected by anti-HA and anti-FLAG antibodies, respectively. (C) Quantification of puromycin incorporation assay shown in B. (D) The 293T cells were transfected for 24 h and puromycin labeled for 15 min. Puromycin incorporation was determined by immunoblotting. (E) The 293T cells were cotransfected with HA-tagged NSP14 and FLAG-tagged NSP10 or its mutants for 24 h. Cell lysates were subjected to IP using mouse anti-HA antibody and followed by immunoblotting with rabbit anti-HA and anti-FLAG antibodies. (F) The 293T cells were transfected for 24 h and puromycin labeled for 15 min. Puromycin incorporation was determined by immunoblotting. For C, D, and F, data are shown as mean ± SD of three biological repeats. *P < 0.05, **P < 0.01, ***P < 0.001 by unpaired Student’s t test.