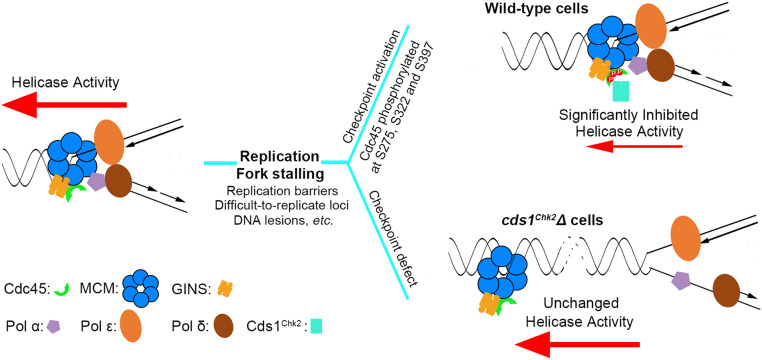
Fig. 7.
Cds1Chk2 phosphorylates Cdc45 to inhibit CMG helicase activity, reduce fork speed, and prevent physical separation of the CMG helicase and DNA polymerases in stalling/stalled replication forks. This results in the stabilization of stalling replication forks. (Left) As replication forks traverse DNA, the polymerases act coordinately with the CMG helicase to synthesize the leading and lagging strands. When replication polymerases are impeded, a small amount of ssDNA is revealed, which activates the intra-S phase ATR-dependent checkpoint pathway (Top, Right). Activated Cds1Chk2 phosphorylates the Cdc45 subunit of the CMG replicative helicase complex on S275, S322, and S397. This results in a severe reduction of CMG helicase activity and prevents the physical uncoupling of strand separation and DNA polymerization, thus stabilizing the stalling replication fork. (Bottom, Right) In checkpoint-deficient cells, CMG helicase activity is not inhibited, which results in DNA unwinding proceeding faster than DNA polymerization, the exposure of excessive ssDNA, and fork collapse.