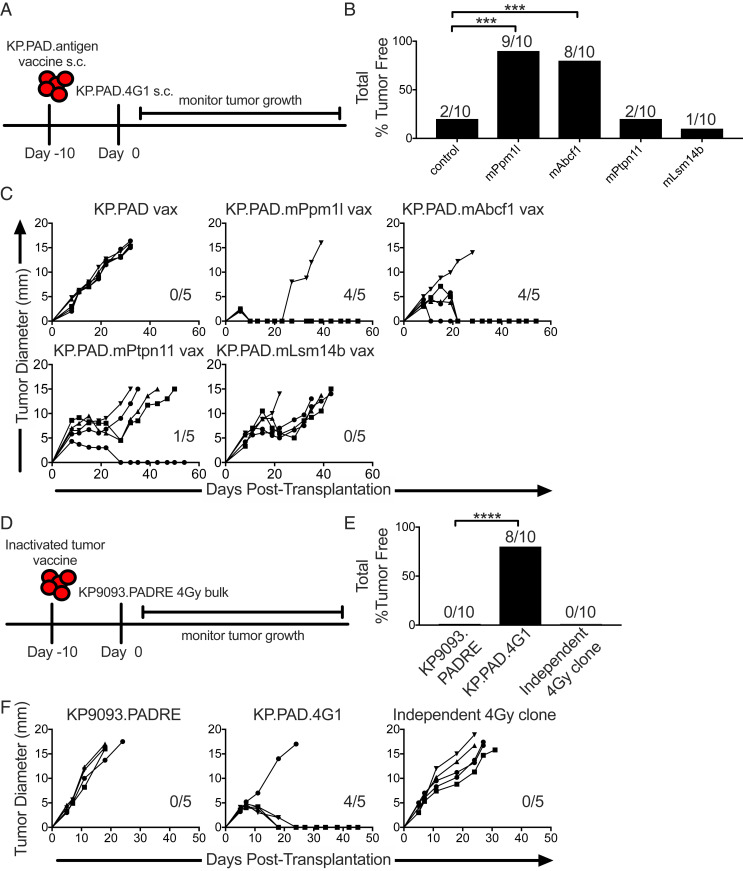
Fig. 5.
Targeting radiation-induced mutations through vaccination is sufficient to promote tumor rejection. (A) WT mice were injected with inactivated tumor cell vaccines consisting of KP9093.PADRE cells ectopically expressing single, radiation-induced neoantigens identified from KP.PAD.4G1 10 d prior to KP.PAD.4G1 tumor challenge. s.c., subcutaneous. (B) Cumulative percentage of mice that rejected KP.PAD.4G1 following treatment as in A. (C) Individual KP.PAD.4G1 tumor growth curves from mice treated as in A. Numbers indicate the number of mice that rejected KP.PAD.4G1 tumors. Representative data from three independent experiments are shown; n = 5 per group. (D) WT mice were treated with parental KP9093.PADRE, KP.PAD.4G1, or an antigenically irrelevant clone derived from an independent 4-Gy-treated bulk cell line as inactivated tumor vaccines followed by challenge with the bulk KP9093.PADRE cell line from which KP.PAD.4G1 was derived 10 d later. (E) Cumulative percentage of mice that rejected the 4-Gy-irradiated KP9093.PADRE bulk line following treatment as in D. (F) Representative data are shown as individual bulk 4-Gy-irradiated KP9093.PADRE tumor growth curves as in D; n = 5 per group. Numbers in the graphs indicate the number of mice that rejected their tumors. ***P < 0.001, ****P < 0.0001.