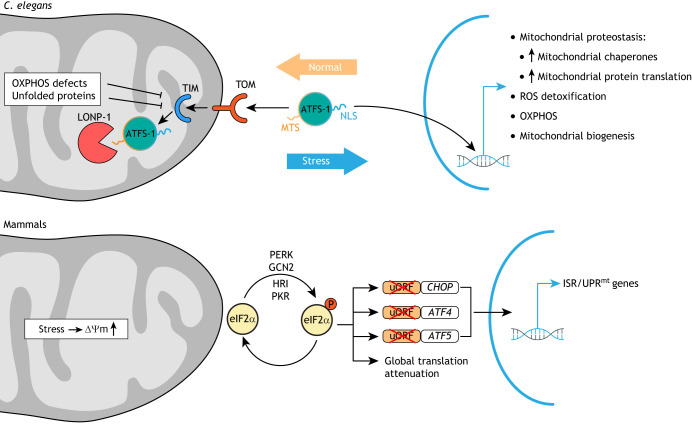
Fig. 3.
Models of UPRmt pathways in C. elegans and mammals. In C. elegans, the mitochondrial unfolded protein response (UPRmt) is regulated by the subcellular localization of the transcription factor ATFS-1, which harbors both a mitochondrial targeting sequence (MTS) and a nuclear localization signal (NLS). ATFS-1 is normally efficiently imported into mitochondria through the TOM-TIM mitochondrial translocation complexes and degraded by the protease LONP-1. If ATFS-1 cannot be imported due to mitochondrial stress, ATFS-1 translocates, via the NLS, into the nucleus to activate a broad transcriptional stress response. In mammals, no direct homolog of ATFS-1 has been identified. Rather, the integrated stress response (ISR) is activated via the translation initiation factor eIF2α. Under mitochondrial stress conditions, eIF2α is phosphorylated by four different kinases (PERK, GCN2, HRI or PKR) that are activated by different stimuli. This leads to global translational attenuation, and, at the same time, translation of the transcription factors CHOP, ATF4 and ATF5. This occurs due to skipped translation of the upstream open reading frames (uORFs) that normally inhibit translation of the downstream CHOP, ATF4 and ATF5 coding sequences. OXPHOS, oxidative phosphorylation; ΔΨm, membrane potential.