Fig. 4.
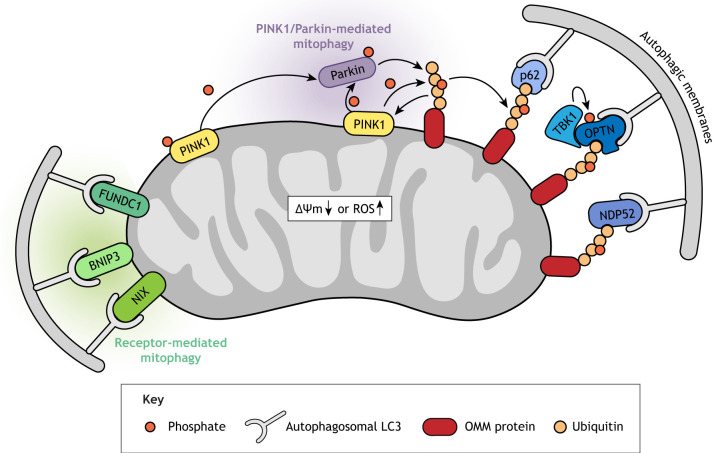
Model of ubiquitin-dependent and -independent mitophagy pathways. Mitochondrial stress stabilizes PINK1 on the outer mitochondrial membrane (OMM). PINK1 is activated by autophosphorylation and then phosphorylates Parkin and ubiquitin, both of which activate Parkin's E3 ligase activity. Parkin ubiquitinates several OMM proteins, and the resulting poly-ubiquitin chains in turn serve as additional phosphorylation targets for PINK1, creating a feed-forward loop. The phosphorylated poly-ubiquitin chains trigger the recruitment of the ubiquitin-binding adaptor proteins OPTN, NDP52 and p62, which initiate autophagosome formation by directly binding to the autophagosomal light chain 3 (LC3) protein through their LC-interacting region motifs. OPTN's affinity for ubiquitin chains is enhanced by its phosphorylation, and TANK binding kinase 1 (TBK1). Receptor-mediated mitophagy relies on various OMM proteins including BNIP3, NIX and FUNDC1, which directly interact with LC3 to mediate autophagosome formation.