FIGURE 6.
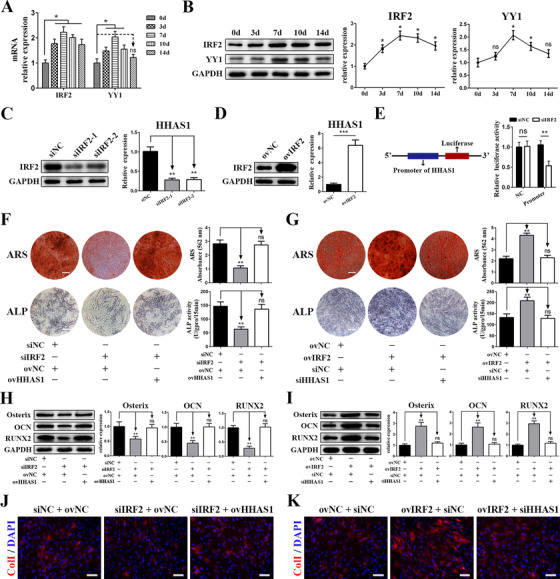
IRF2 promotes the transcription of HHAS1 during BMSC osteogenic differentiation. (A) The mRNA levels of predicted transcription factor, IRF2, and YY1 in BMSCs during osteogenic differentiation. The levels of IRF2 and YY1 increased and reached a maximum on day 7. (B) The protein levels of IRF2 and YY1 in BMSCs during osteogenic differentiation. (C) The efficiency of siRNAs targeting IRF2 was measured by western blotting (left), and HHAS1 expression was decreased by IRF2 siRNAs (right). (D) The efficiency of IRF2 overexpression was measured by western blotting (left), and HHAS1 expression was increased by IRF2 overexpression (right). (E) The HHAS1 promoter was inserted into the 5′ end of the luciferase gene. Silencing IRF2 significantly inhibited the luciferase activity of the promoter group. (F) Silencing IRF2 markedly decreased the ARS staining, ALP staining and ALP activity of BMSCs, while overexpressing HHAS1 abrogated these effects (scale bar = 200 μm). (G) Overexpressing IRF2 significantly enhanced the ARS staining, ALP staining and ALP activity of BMSCs, while silencing HHAS1 abrogated these effects (scale bar = 200 μm). (H) Silencing IRF2 significantly reduced the protein levels of Osterix, OCN, and RUNX2 in BMSCs, while overexpressing HHAS1 abrogated these effects. (I) Overexpressing IRF2 significantly enhanced the protein levels of Osterix, OCN, and RUNX2 in BMSCs, while silencing HHAS1 abrogated these effects. (J) Silencing IRF2 significantly impaired the fluorescence signal of ColI in BMSCs, while overexpressing HHAS1 abrogated these effects (scale bar = 50 μm). (K) Overexpressing IRF2 significantly strengthened the fluorescence signal of ColI in BMSCs, while silencing HHAS1 abrogated these effects (scale bar = 50 μm). The data are presented as the mean ± SD (n = 10, determined by independent‐sample t‐tests). All experiments were performed three independent times, ns = not statistically significant, *p < 0.05, **p < 0.01, ***p < 0.001
