Fig. 1.
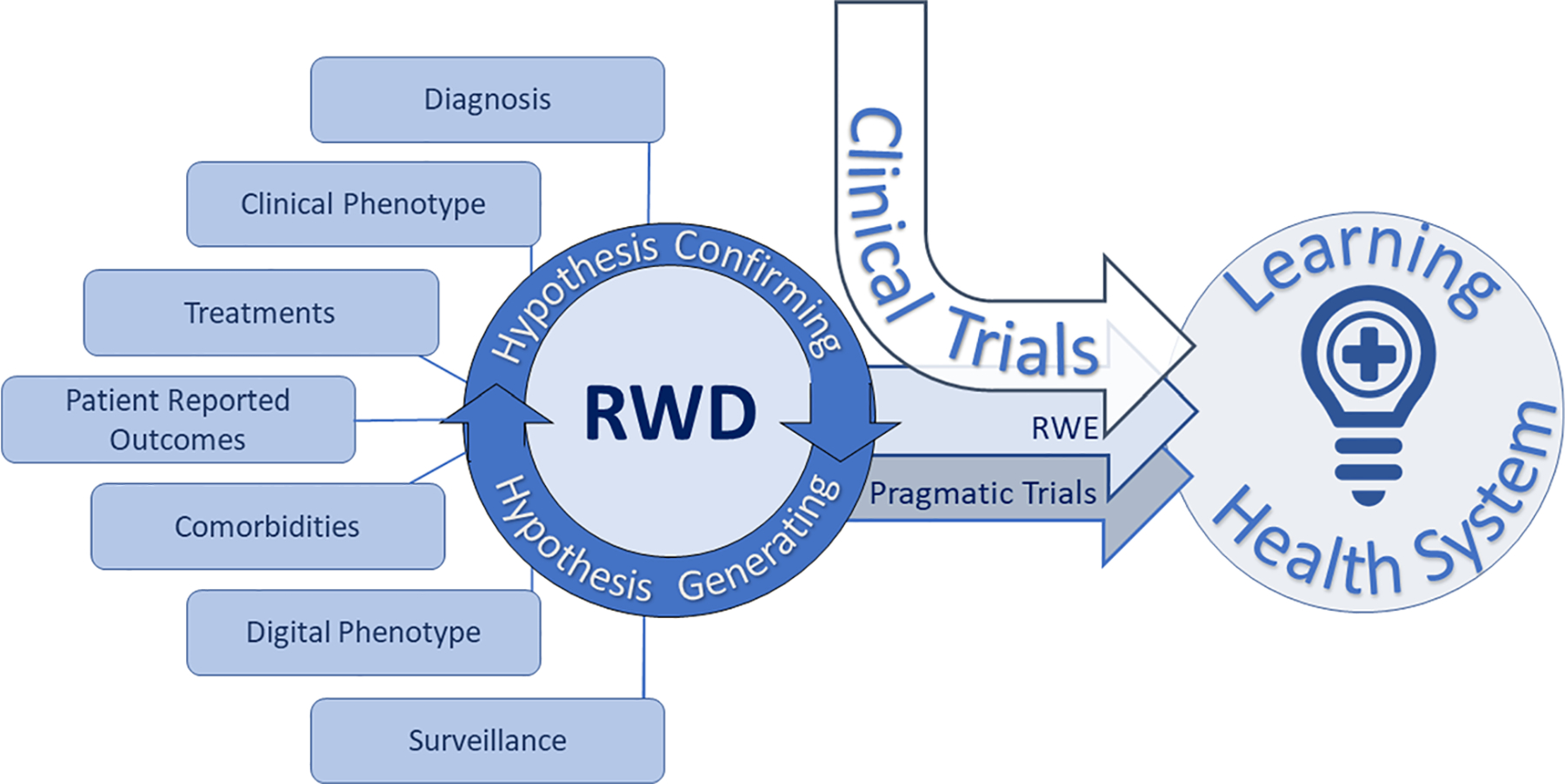
Real-world evidence (RWE) is derived from real-world data (RWD) systematically obtained from a multitude of sources loosely defined as patient information obtained outside of traditional research settings. RWE can expand the impact of prospective interventional clinical trials through evidence generation of the patient experience in conditions most similar to everyday healthcare situations (i.e., patients with additional comorbidities treated in routine clinical environments). Pragmatic clinical trials are a study type aimed at showing impact of an intervention in broad patient populations with minimal deviation in standard practice and are thus well-suited to RWE-based analysis. Classical clinical trials, RWE, and pragmatic trials inform learning healthcare systems
