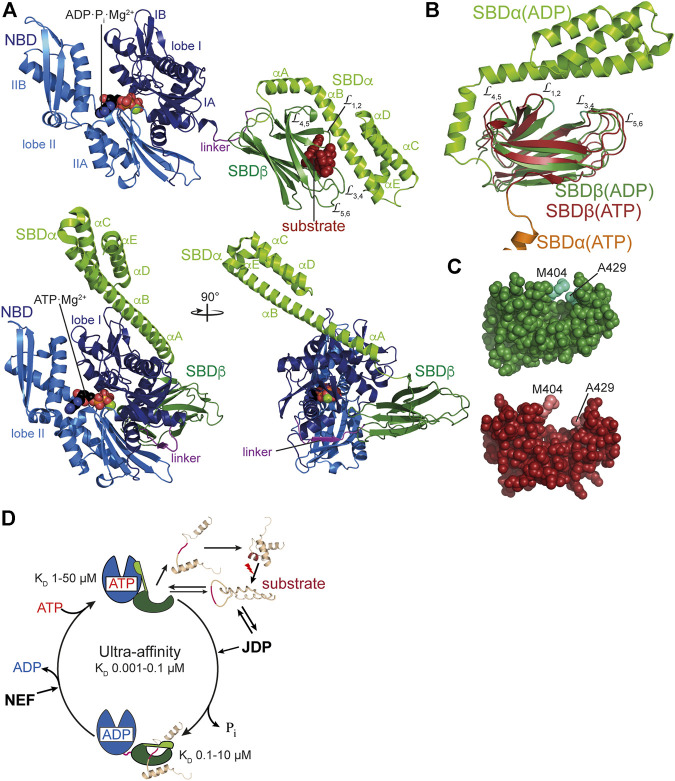
FIGURE 2.
Structure and functional cycle of Hsp70s. (A), Cartoon representation of DnaK in the ADP·Pi·Mg2+-bound, SBD-closed and domain-undocked conformation (upper panel; PDB ID 2KHO (Bertelsen et al., 2009)) and ATP·Mg2+-bound, SBD-open, domain-docked conformation [lower panels in two orientations; 4B9Q (Kityk et al., 2012)]. NBD lobe I (subdomains IA and IB), dark blue; NBD lobe II (subdomains IIA and IIB), marine blue; conserved linker, magenta; SBDβ, dark green; SBDα, chartreuse; ADP and ATP in space-filling representation colored according to atoms with carbon, black, oxygen, red, nitrogen blue and phosphorus, orange, Mg2+, green; substrate peptide, dark red in space-filling representation. (B), Overlay of the structures of the SBD of the ADP-bound, closed [SBDβ, dark green and SBDα, chartreuse; 1DKX (Zhu et al., 1996)] and the ATP-bound, open conformation [SBDβ, dark red and SBDα, orange, cut for space reasons; 4B9Q (Kityk et al., 2012)]. Substrate enclosing loops L1,2, L3,4, L4,5, and L5,6 are labeled. (C), space-filling representation of the crystal structure of the SBDβ in the closed, substrate-bound conformation (upper panel, dark green), and the open conformation in the ATP-bound state (lower panel, dark red); arch forming residues M404 and A429 are indicated. (D), ATPase cycle of Hsp70s. Partially folded or misfolded substrate polypeptides associate with and dissociate from Hsp70 with high rates in the ATP-bound open conformation. Substrates may also interact with the J-domain protein (JDP) co-chaperone. Substrate and JDP synergistically trigger ATP hydrolysis and transition to the closed, domain-undocked conformation. During this process substrate unfolding may occur. Alternatively or in addition, Hsp70 may select the more unfolded species from a equilibrium of different conformations. At physiological ATP concentrations nucleotide exchange is rate-limiting for substrate release. Nucleotide exchange factors (NEF) catalyze ADP release, and ATP rebinding stimulates substrate release that subsequently might fold into the native state or might rebind to Hsp70 for another folding cycle. Dark red indicate Hsp70 binding site. KD values for typical high-affinity binding peptides to ADP and ATP bound states are indicated. Association of the substrate to the ATP-bound state with subsequent ATP hydrolysis creates a non-equilibrium situation called ultra-affinity (De Los Rios and Barducci, 2014).