Abstract
Purpose: To evaluate pharmacokinetic parameters and ocular hypotensive effects of cromakalim prodrug 1 (CKLP1) in normotensive large animal models.
Methods: Optimal CKLP1 concentration was determined by dose response and utilized in short- (5–8 days) and long-term (60 days) evaluation in hound dogs (n = 5) and African Green Monkeys (n = 5). Blood pressure was recorded 3–5 times per week with a tail cuff. Concentrations of CKLP1 and the parent compound levcromakalim were assessed in hound dog plasma and select tissues by LC-MS/MS after bilateral ocular treatment with CKLP1 for 8 days. Pharmacokinetic parameters were calculated from days 1, 4, and 8 data. After necropsy, histology was assessed in 43 tissue samples from each animal.
Results: In hound dogs and African Green monkeys, 10 mM CKLP1 (optimal concentration) significantly lowered intraocular pressure (IOP) by 18.9% ± 1.1% and 16.7% ± 6.7%, respectively, compared with control eyes (P < 0.05). During treatment, no significant change in systolic or diastolic blood pressure was observed in either species (P > 0.1). Average values for half-life of CKLP1 was 295.3 ± 140.4 min, Cmax, 10.5 ± 1.6 ng/mL, and area under the concentration vs. time curve (AUClast) 5261.4 ± 918.9 ng·min/mL. For levcromakalim, average values of half-life were 96.2 ± 27 min, Cmax 1.2 ± 0.2 ng/mL, and AUClast 281.2 ± 110.8 ng·min/mL. No significant pathology was identified.
Conclusions: CKLP1 lowered IOP in hound dogs and African green monkeys with no effect on systemic blood pressure. Ocular topical treatment of CKLP1 showed excellent tolerability even after extended treatment periods.
Keywords: CKLP1, levcromakalim, KATP channel openers, IOP, dogs, nonhuman primates
Introduction
Glaucoma, an optic neuropathy characterized by progressive loss of retinal ganglion cells, is the leading cause of permanent blindness.1,2 All current treatment strategies (pharmacological or surgical) are aimed at lowering intraocular pressure (IOP), which is the only modifiable risk factor of glaucoma. Unfortunately, first-line glaucoma drugs such as timolol and latanoprost can become ineffective in up to 30% of glaucoma patients3,4 and all therapies can cause mild to severe side effects.5–12 Therefore, the development of novel, safe, and effective glaucoma therapies is still a common goal for the ophthalmic community.
Our laboratory has identified ATP-sensitive potassium (KATP) channel openers (eg, diazoxide and levcromakalim) as a new class of ocular hypotensive agents.13–17 Owing to the relative insolubility of commercially available KATP channel openers, we developed a water-soluble direct phosphate-linked prodrug called cromakalim prodrug 1 (CKLP1, chemically referred to as sodium [(3S,4R)-6-cyano-2,2-dimethyl-4-(2-oxopyrrolidin-1-yl)-chroman-3-yl phosphate] based on the structure of the KATP channel opener levcromakalim.16–19 After topical application, CKLP1 is converted in vivo, to its active moiety levcromakalim by alkaline phosphatases in the eye.18,20
Our studies in normotensive mice and rabbit models indicate that CLKP1 retains the ocular hypotensive properties of the parent compound levcromakalim.16–19 Furthermore, data published from our laboratory show that CKLP1 lowers IOP by targeting the distal outflow region of the conventional outflow pathway and has a lowering effect on episcleral venous pressure.19 Because of this mode of action, CKLP1 lowers IOP in an additive manner when used in combination with conventional glaucoma therapeutics such as latanoprost, timolol, and rho kinase inhibitors.19
In light of this, CKLP1 has emerged as a strong candidate for further assessment in human clinical trials. However, IOP and pharmacodynamics data are required in large animal models before obtaining this goal. Here we describe the IOP lowering ability of CKLP1 in hound dogs and African green monkeys. In addition, after bilateral topical ocular application in hound dogs, we characterize pharmacokinetic parameters of CKLP1 and levcromakalim in plasma, assess their pharmacodynamics in tissues, and perform histological assessment of local and systemic tissue samples in a masked manner.
Methods
Animal care
All animal experiments were preapproved either by the Institutional Animal Care and Use Committee at Mayo Clinic, Rochester, MN (for hound dogs) or the Animal Care and Use Committee at Wake Forest School of Medicine, University of Wake Forest, Winston-Salem, NC (for African green monkeys). Research protocols adhered to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the tenets of the ARVO Statement for the Use of Animals in Vision Research. All procedures involving African green monkeys strictly adhered to local and federal laws and standards of the Department of Health and Human Services, and guidelines established by the Wake Forest Institutional Animal Care and Use Committee.
Healthy female mixed-breed hound dogs (n = 5; average age, 4.5 ± 0.7 years, range, 3–5 years; average weight, 30.2 ± 5.4 kg, range, 25–37 kg) were obtained from approved institutional vendors. Animals were socially housed in compatible pairs in climate-controlled rooms according to accepted practices adopted by the Mayo Clinic Department of Comparative Medicine. Kennels were cleaned daily and hound dogs were evaluated for general health at least 3 times per week. Animals were removed from cages during IOP and blood pressure measurements, both of which lasted for <10 min. Animals were returned to social housing immediately after procedures. Canine food and water were available ad libitum.
Healthy African green monkeys (n = 5; average age, 12.3 ± 1.5 years, range, 10.4–14.3 years; average weight, 8.0 ± 0.5 kg, range, 7.7–8.9 kg) without ocular and systemic disorders were selected from the existing multigenerational, pedigreed vervet research colony at Wake Forest University. Animals were initially housed in large enclosures with both indoor and outdoor spaces in groups mimicking natural social composition in the wild. Monkeys had free access to commercial monkey food and water. Animals were brought out of their enclosures at 8 am on the morning of their scheduled IOP and blood pressure measurement procedures and returned to cages as soon as they recovered from anesthesia.
IOP, blood pressure, and CKLP1 treatment in hound dogs
IOP was measured 3 times each day at times that corresponded to 1, 4 and 23 h post-treatment. For IOP measurements, dogs were temporarily isolated in individual cages and gently restrained by laboratory personnel while a second operator used a handheld rebound tonometer (Icare Tonovet, Colonial Medical Supply, Franconia, NH). Owing to the minimal and fleeting contact of the tonometer probe to the cornea, there was no need for any topical anesthetic while recording IOP with this instrument. The average of the measurements at 3 time points on any given day was recorded as the daily IOP. To minimize stress, animals were subjected to sham IOP measurements for 3 days before the start of actual experiments.
For measuring blood pressure, dogs were placed in a sling and partially suspended to reduce weight on their limbs. Blood pressure was measured using an automated sphygmomanometer and a tail cuff that was attached at the base of the tail.
Three dogs were selected for the dose–response study. After baseline IOP measurements were obtained and recorded (3 consecutive days before treatment), one eye of each dog was treated with a 50 μL bolus of 5 mM CKLP1 (synthesized as [3S,4R]-2 as previously described),18 whereas the contralateral eye was treated with 10 mM CKLP1, once daily for 5 consecutive days. After 5 days, the eye that received 5 mM CKLP1 was treated with 15 mM CKLP1, whereas the eye with 10 mM CKLP1 received 20 mM CKLP1. IOPs were measured every day at times corresponding to 1, 4, and 23 h post-treatment. The various doses of CKLP1 (dissolved in PBS) were added as a 50 μL bolus once daily in the morning at ∼9:30 am. For all experiments, the right eye was used as control, whereas the left eye was selected as the treatment eye.
For the extended dose study, baseline IOPs were measured 3 times daily for 5 consecutive days. The average of the 3 measurements was recorded as the daily IOP and averaged over the 5 days for the final pretreatment value. After baseline IOP measurements, dogs (n = 5) were treated with 10 mM CKLP1 in one eye, whereas the contralateral eye received vehicle (PBS). IOP was measured at least 3 times every week at times corresponding to 1, 4, and 23 h post-treatment. Blood pressure was measured 3 times each week at the 4-h post-treatment time point.
IOP, blood pressure, and CKLP1 treatment in monkeys
African green monkeys were removed from their enclosures at 8 am each day and lightly anesthetized with ketamine (14 mg/kg), injected intramuscularly. IOP was measured using a handheld tonovet tonometer (as described previously) while the animal was held upright. Measurement time points corresponded to 20 min and 23-h post-treatment. Blood pressure was measured in supine monkeys, utilizing a tail cuff and an automated sphygmomanometer.
For treatment days, 10 mM CKLP1 (dissolved in PBS) was added to one eye of each monkey in a 50 μL bolus, once daily, for 7 consecutive days, whereas the contralateral eye received a 50 μL bolus of vehicle (PBS). All treatments were performed in the morning between 8:30 am and 9 am.
Pharmacokinetic studies
Hound dogs (n = 3) were treated bilaterally with 10 mM CKLP1 (50 μl) once daily for 8 days, whereas the remaining 2 dogs were topically treated with the vehicle (PBS) in both eyes. Approximately 3 mL of blood was collected from treated and control animals on treatment days 1, 4, and 8 at 8 different time points (5, 15, 30, 60 min, 2, 4, 8, and 24 h) into heparin-coated blood collection tubes (BD vacutainer; Becton, Dickinson and Company, Franklin Lakes, NJ) following ocular instillation of CKLP1. Plasma was separated from the blood by centrifugation at 2,000 rpm for 5 min. After 4–5 additional days of CKLP1 and vehicle treatment, all dogs were euthanized and necropsied to collect ocular and systemic tissue samples (see Supplementary Fig. S1 with images representing histology of all tissues) and urine.
For ocular tissue collection, eyes were enucleated and aqueous humor, vitreous humor, trabecular meshwork/aqueous plexus, optic nerve, ciliary body, iris, retina, and cornea were isolated and stored at −80°C. While collecting tissues during necropsy, portions of the heart, kidney, lung, brain, liver and skeletal muscle were isolated and flash frozen for pharmacodynamics analysis, while the remaining tissue samples were immediately fixed in 10% neutral buffered formalin.
Analysis of blood chemistries
Immediately before euthanasia of the dogs, blood plasma was collected as described previously and stored in a −80°C freezer. The plasma was used to analyze general blood chemistry parameters of all dogs using a pHOx Ultra analyzer (Nova Biomedical, Waltham, MA), according to the manufacturer's protocol and utilizing an automated internal quality assurance system to validate test results.
Bioanalytical methods for detecting CKLP1 and levcromakalim
CKLP1 and levcromakalim concentrations in the biological samples (fluids and tissues) were determined by an established LC-MS/MS-based assay.20 The lower limit of detection of CKLP1 and levcromakalim was 5 and 1 ng/mL, respectively. Immediately before analysis, tissues were thawed and their wet weight was measured. PBS was added at double the tissue volume, and the tissue was homogenized in a rotor stator homogenizer (Bio-Gen Pro200; PRO Scientific, Oxford, CT) for 30 s. In brief, CKLP1, levcromakalim, and flavopiridol (internal standard) were separated on a Waters (Milford, MA) Acquity UPLCBEH C18 column (1.7 μm, 2.1 × 50 mm) coupled with an Agilent EC-C18 precolumn (2.7 μm, 2.1 × 5 mm).20 Detection was accomplished using positive electrospray ionization with multiple-reaction monitoring (MRM). The MRM precursor and product ions were monitored at m/z 367 > 86, 287 > 86, and 402 > 341 for CKLP1, levcromakalim, and flavopiridol (internal standard), respectively. Data were acquired and analyzed using Waters MassLynx v4.1 software.20
Histology
Tissues collected and fixed during necropsy were processed into paraffin blocks, sectioned, and stained with hematoxylin and eosin as previously described.20 All sections were evaluated by a board-certified veterinary pathologist in a masked manner.
Statistics
Values are expressed as mean ± standard deviations. Group mean values within the same animal were compared using paired t-tests. Mean values for more than 2 groups (dose–response studies) were compared using one-way analysis of variance (ANOVA) followed by pairwise t-tests to account for dependence between eyes of same animals when applicable. Statistical tests were performed using JMP software or the data analysis add-on feature of Microsoft Excel.
Results
Determination of optimal dose of CKLP1 for lowering IOP in hound dogs
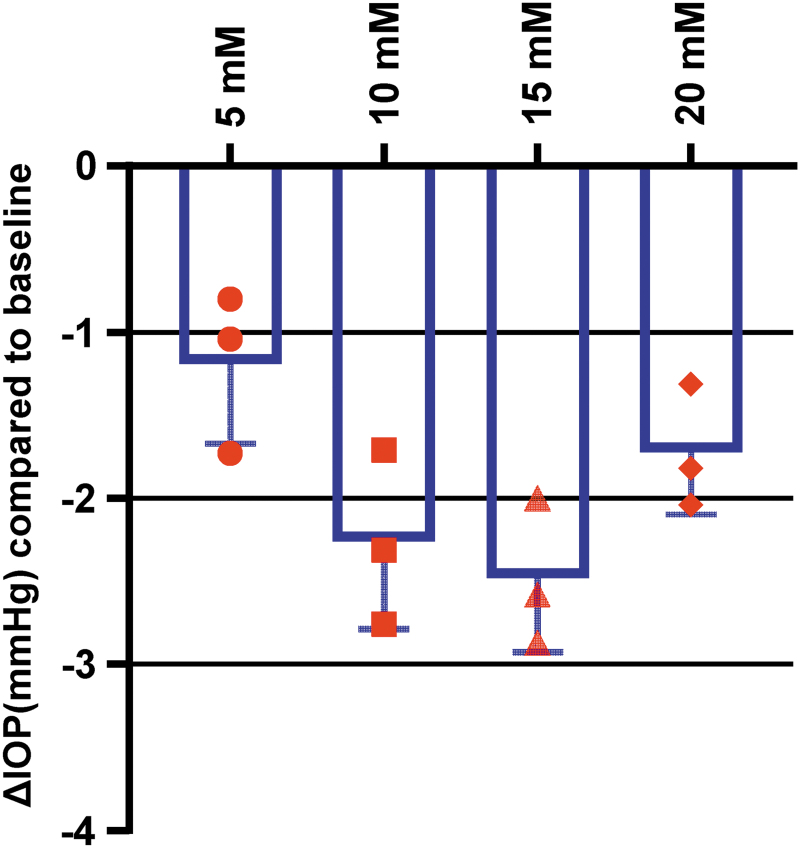
To determine the optimal dose of CKLP1 for subsequent pharmacokinetic studies, hound dogs (n = 3) were treated with 4 different concentrations of CKLP1 (5, 10, 15, and 20 mM), once daily for 5 consecutive days. All CKLP1 concentrations showed a significant IOP lowering (P ≤ 0.01) with greatest effect observed with 10 mM (2.3 ± 0.5 mmHg) and 15 mM (2.5 ± 0.4 mmHg) (Fig. 1). No statistical difference in IOP reduction was noted between the 10 and 15 mM doses (P = 0.57). To utilize the lowest dose concentration with effective IOP reduction, a 50 μL bolus of 10 mM CKLP1 was selected as the optimal dose for all subsequent experiments.
FIG. 1.
Dose response of CKLP1 in hound dogs. Dose–response studies with CKLP1 showed all concentrations lowered IOP significantly. Statistically, both 10 and 15 mM concentrations had the greatest reduction in IOP, although no difference was noted between the 2 concentrations. Therefore, the 10 mM concentration was selected for all subsequent experiments. Symbols in red represent the average of IOP readings from individual dogs treated with different doses of CKLP1. CKLP1, cromakalim prodrug 1; IOP, intraocular pressure.
Effect of CKLP1 on IOP and systemic blood pressure in hound dogs
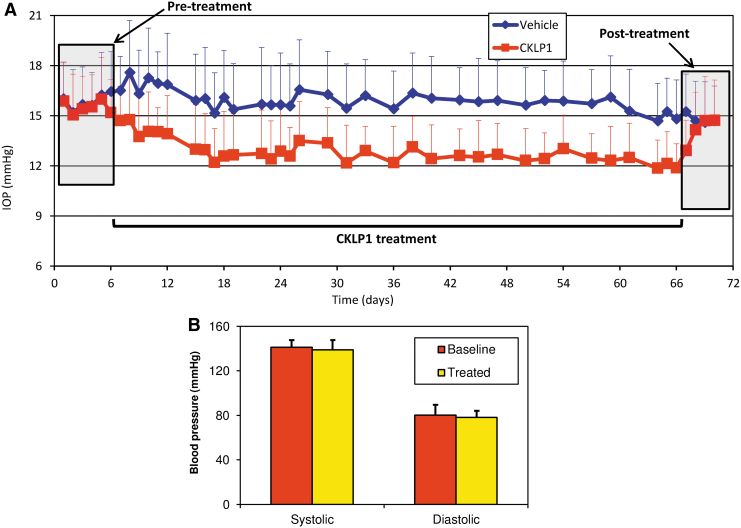
To establish baseline IOPs in hound dogs, 3 measurements were performed daily for 5 consecutive days. Average baseline values for the right (vehicle) and left (treated) eyes were 15.7 ± 2.3 and 15.6 ± 2.1 mmHg, respectively. To evaluate the effect of CKLP1 on IOP lowering, hound dogs (n = 5) were treated with 10 mM CKLP1 in one eye and vehicle (PBS) in the contralateral eye once daily for 61 consecutive days. Over the course of the experiment, the average IOP in the vehicle-treated eye was 16.0 ± 2.4 mmHg, whereas in the treated eye it was significantly lower (12.9 ± 2.0 mmHg, P < 0.001; Fig. 2A). On average, IOP was reduced by 18.9% ± 1.3% (reduction of 3.0 ± 0.5 mmHg; P < 0.001) over the entire treatment period. When compared by one-way ANOVA, no statistical differences were found in the IOP values of individual post-treatment time points of 1, 4, and 23 h on IOP recording days (P = 0.10; F < Fcrit).
FIG. 2.
IOP and blood pressure in hound dogs after treatment with CKLP1. (A) Once-daily treatment of 10 mM CKLP1 caused sustained IOP reduction over a treatment period of 61 consecutive days in hound dogs with excellent tolerability and no observable ocular side effects. Each data point represents average absolute IOP values in mmHg (y axis) ± standard deviation for CKLP1 and vehicle- (PBS) treated eyes for a given day, plotted against time (x-axis). (B) CKLP1 treatment did not cause any changes in average systolic or diastolic blood pressures compared with baseline values.
In addition to IOP, the effect of CKLP1 treatment on blood pressure and several safety parameters were assessed. No significant change in systolic (baseline, 141.0 ± 6.7; treatment, 138.9 ± 9.5; P = 0.56) or diastolic (baseline, 80.1 ± 8.9; treatment, 78.1 ± 5.9; P = 0.76) pressure was observed during the treatment period (Fig. 2B). Hound dogs were also evaluated for eye redness, swelling of the eye or eyelids, unusual discharge from eye, and overall food intake. No notable findings in these parameters were identified suggesting that CKLP1 did not produce acute or chronic side effects after once daily topical application for >2 months.
Effect of CKLP1 on IOP and systemic blood pressure in African green monkeys
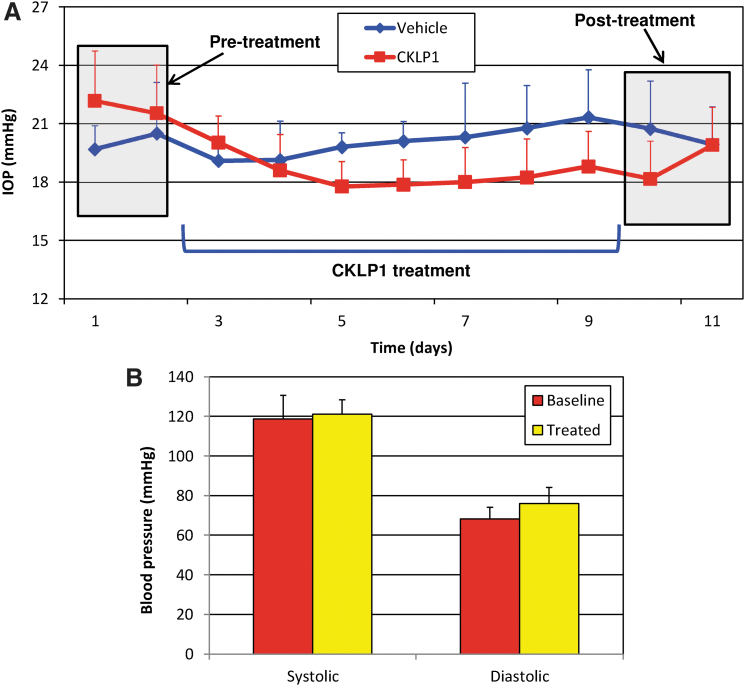
To further validate the IOP lowering effects of CKLP1 in large animal models, we treated one eye of 5 African green monkeys with 10 mM CKLP1, whereas the contralateral eye received vehicle (PBS). Baseline IOP in control and treated eyes were 20.1 ± 1.8 mmHg and 21.9 ± 2.5 mmHg, respectively. After treatment, the eye that received CKLP1 had an IOP reduction of 3.8 ± 1.8 mmHg compared with baseline (P = 0.01), which corresponded to a 16.7% ± 6.7% change in IOP. In contrast, the vehicle-treated eyes showed an increase in IOP of 0.1 ± 1.0 mmHg, which was not statistically different from baseline (P = 0.80; Fig. 3A). Comparison of the IOP values at early and late time points did not yield statistically significant differences (P = 0.6).
FIG. 3.
IOP and blood pressure in African green monkeys after CKLP1 treatment. (A) Once-daily treatment with 10 mM CKLP1 lowered IOP in African green monkeys. IOP returned to baseline after withdrawal of treatment. No side effects were observed during the course of the treatment. (B) Daily treatment with 10 mM CKLP1 for a period of 7 days had no effect on systolic or diastolic blood pressure in African green monkeys.
Similar to the hound dogs, topical ocular instillation of CLKP1 did not have any effect on systemic blood pressure compared with baseline. Average baseline systolic pressure was 118.7 ± 12.0 mmHg, which slightly increased to 121.1 ± 7.3 mmHg after treatment (P = 0.6). Likewise, there was no significant change in diastolic pressure after CKLP1 treatment (76 ± 8.2 mmHg) compared with baseline (68.1 ± 6.0 mmHg; P = 0.13; Fig. 3B).
Analysis of pharmacokinetic parameters of CKLP1 and levcromakalim in hound dogs
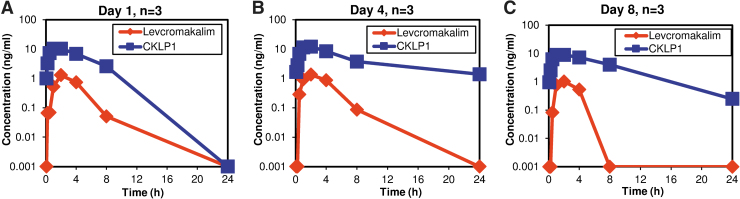
After completing the long-term evaluation of the effects of CKLP1 on IOP and blood pressure, the hound dogs were allowed a washout period of 2 months, after which, they were used to assess pharmacokinetic parameters. For this, the hound dogs were treated with 50 μL topical eye drops of 10 mM CKLP1 (n = 3) or vehicle (PBS, n = 2) in both eyes, once daily for 8 days and blood was collected at various time points after treatment on days 1, 4, and 8. Pharmacokinetic analysis of these samples showed characteristic distribution, absorption, and elimination profiles of CKLP1 and levcromakalim (Fig. 4A–C). Average maximum concentration of CKLP1 (10.6 ± 1.6 ng/mL) in plasma was obtained within 80.0 ± 34.6 min after topical dose. Average maximum concentration of levcromakalim (1.2 ± 0.2 ng/mL) occurred at 120 min. The half-lives of CKLP1 were 180.5, 451.8, and 253.7 min on days 1, 4, and 8 (average 295.3 ± 140.4 min). Half-lives of the active moiety levcromakalim on those same days were 74.3, 87.8 and 126.4 min (average 96.2 ± 27.0 min). Average AUClast for CKLP1 (5261.4 ± 918.9 ng*min/mL) was 22.4-fold greater than levcromakalim (233.0 ± 102.8 ng·min/mL), indicating a possible slow release of CLKP1 from an internal tissue source in the animals. This is further indicated by a longer Tlast (time when drug was last detected in plasma) of CKLP1 on days 4 and 8 compared with day 1 (Table 1).
FIG. 4.
Pharmacokinetic analysis of CKLP1 and levcromakalim. Graphs of day 1 (A), day 4 (B), and day 8 (C) indicate conversation of CKLP1 to levcromakalim along with characteristic absorption and elimination profiles of the drugs in plasma. Analysis of the data from these graphs was used to define the various PK parameters described in Table 1.
Table 1.
Pharmacokinetic Parameters of Cromakalim Prodrug 1 and Levcromakalim in Plasma of Hound Dogs
| CKLP1 |
Levcromakalim |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Half-life (min) | Tmax (min) | Cmax (ng/mL) | Tlast (h) | Clast (ng/mL) | AUClast (ng·min/mL) | Half-life (min) | Tmax (min) | Cmax (ng/mL) | Tlast (h) | Clast (ng/mL) | AUClast (ng·min/mL) | |
| Day 1 | 180.5 | 60.0 | 10.3 | 480 | 2.6 | 4,373.8 | 74.3 | 120.0 | 1.3 | 480 | 0.1 | 305.6 |
| Day 4 | 451.8 | 120.0 | 12.2 | 1,441 | 1.4 | 6,208.6 | 87.8 | 120.0 | 1.4 | 480 | 0.1 | 377.8 |
| Day 8 | 253.7 | 60.0 | 8.9 | 1,450 | 0.3 | 5,201.9 | 126.4 | 120.0 | 1.0 | 240 | 0.5 | 160.3 |
| Average (all days) | 295.3 ± 140.4 | 80.0 ± 34.6 | 10.5 ± 1.6 | 1,123.7 ± 557.5 | 1.41 ± 1.2 | 5,261.4 ± 918.9 | 96.2 ± 27.0 | 120.0 ± 0.0 | 1.2 ± 0.2 | 400.0 ± 138.6 | 0.2 ± 0.3 | 281.2 ± 110.8 |
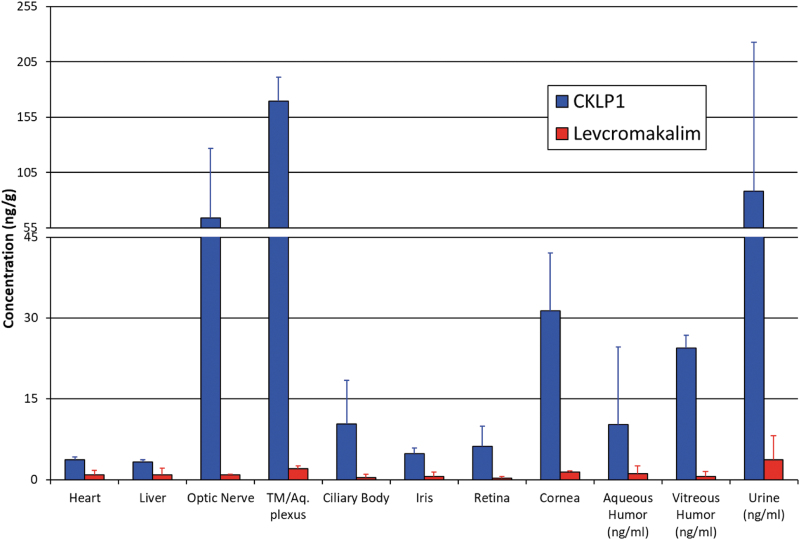
Concentration of CKLP1 and levcromakalim in select ocular and systemic tissues
After blood collection for pharmacokinetic studies, bilateral ocular treatment with CKLP1 (10 mM) continued in the hound dogs for 4–5 additional days (for a total of 12–13 days of once daily treatment). At 23 h after the last treatment, animals were euthanized and select ocular and systemic tissue samples were collected and analyzed for the presence of CKLP1 and levcromakalim by LC-MS/MS. Two of the treated hound dogs showed significant levels of CKLP1 and levcromakalim in their tissues, whereas the levels in the third hound dog were below quantitative limits. Using data from the 2 animals, high concentration of CKLP1 was found in optic nerve (63.8 ± 63.1 ng/g), trabecular meshwork/aqueous plexu (169.5 ± 21.6 ng/g), cornea (31.3 ± 10.8 ng/g), and vitreous humor (24.4 ± 2.4 ng/g; Fig. 5), with lower levels found in ciliary body (10.3 ± 8.1 ng/g), iris (4.8 ± 1.1 ng/g), retina (6.2 ± 3.8 ng/g), and aqueous humor (10.2 ± 14.4 ng/g; Fig. 5). Levcromakalim was also present in these samples but at much lower concentrations. The highest concentrations of levcromakalim were found in the trabecular meshwork/aqueous plexus (2.0 ± 0.5 ng/g) followed by cornea (1.4 ± 0.3 ng/g) and aqueous humor (1.1 ± 1.5 ng/mL). Optic nerve, ciliary body, iris, retina, and vitreous humor also showed levcromakalim although at <1 ng/g.
FIG. 5.
Distribution of CKLP1 and levcromakalim in various ocular and systemic hound dog tissues and fluids. CKLP1 was identified in low concentrations in heart and liver, and in higher concentrations in all ocular tissues analyzed. Trabecular meshwork/aqueous plexus (TM/Aq. plexus), optic nerve, and cornea showed the highest levels of CKLP1 and levcromakalim. Both drugs were excreted in the urine.
In addition to ocular tissues, several systemic tissues were analyzed for CKLP1 and levcromakalim concentrations. Both CKLP1 and levcromakalim were found at low concentrations in the heart (3.7–0.5 ng/g CKLP1; 0.9–0.8 ng/g levcromakalim), kidney (2.7–2.9 ng/g CKLP1; 0.8–1.2 ng/g levcromakalim), and lung (CKLP1 was undetected; 0.3–0.4 ng/g levcromakalim; Fig. 5). Of interest, a high concentration of both CKLP1 (88.0–134.9 ng/mL) and levcromakalim (3.7–4.5 ng/mL) was noted in urine of the treated animals indicating this to be an important route of drug excretion from the body.
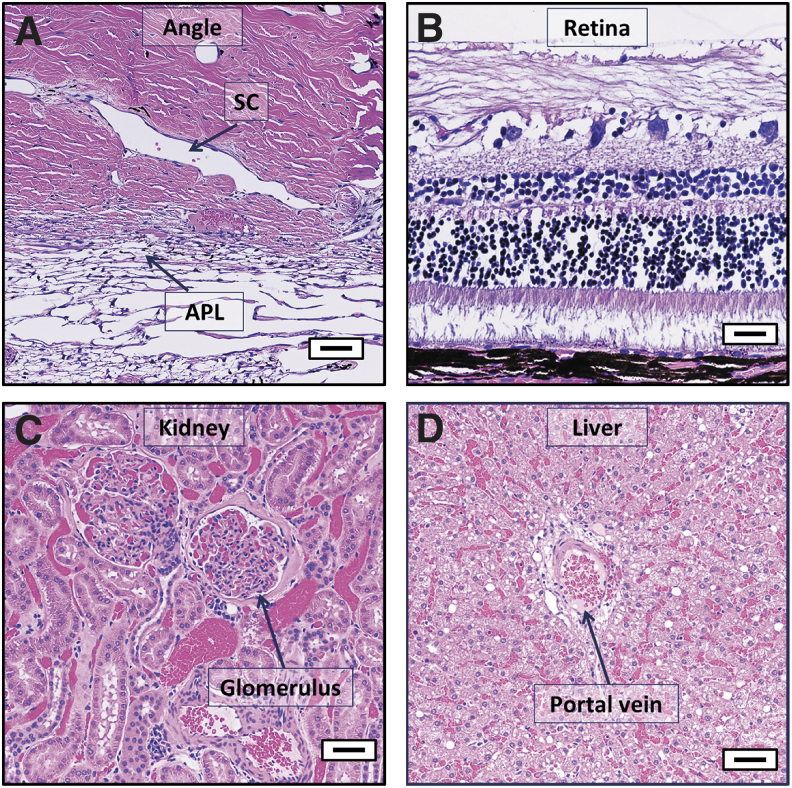
To evaluate local and systemic side effects of bilateral topical administration of CKLP1 to the eyes, additional tissue samples were harvested for histological examination. Some evidence of postmortem autolysis was observed in the eye sections, although accompanying morphological alterations were minimal and consistent with minor background changes characteristic of mixed-breed hound dogs. Overall, of the 43 different tissues evaluated from each hound dog, none of the analyzed tissues showed any significant pathology beyond incidental findings as evaluated in a masked manner by a board-certified veterinary pathologist. Absence of significant pathological changes suggest that treatment with CKLP1 was absent of any observable toxicity. Figure 6A–D shows representative images from select tissues (trabecular meshwork/aqueous plexus, retina, kidney, and liver) treated with CKLP1. Supplementary Figure S1 shows representative images of all the tissues evaluated.
FIG. 6.
Histological assessment of selected hound dog tissues following treatment with CKLP1. Representative tissue sections from trabecular meshwork/aqueous plexus (A), retina (B), kidney (C), and liver (D) of hound dogs treated once daily with CKLP1 for 12–13 days were devoid of any pathological findings, indicating appropriate tolerance of CKLP1. APL, aqueous plexus; SC, Schlemm's canal; Scale bar, 50 μm.
To further evaluate possible effects of topical CKLP1 treatment, blood collected from all hound dogs after 14 days of CKLP1 treatment was used to analyze blood chemistry (Table 2). All values were within normal range for hound dogs except for albumin, which was found to be at a slightly lower concentration in both treated and control animals, compared with the historical range in this species. In addition, no changes were observed in food intake or behavior of the hound dogs during the treatment period. Likewise, the weight of the dogs before and after experiment did not show any significant changes (P > 0.36 for both treated and control groups; Table 3). Together, these results suggest that bilateral eye treatment with CKLP1 was well tolerated and did not result in any ocular or systemic toxicity.
Table 2.
Blood Chemistry of Hound Dogs After Ocular Topical Instillation of 50 mM Cromakalim Prodrug 1
| Average (Control) | Average (Treated) | Normal range (dogs) | |
|---|---|---|---|
| Sodium (Na+) (mM) | 144.5 ± 2.1 | 137.7 ± 8.5 | 139.0–154.0 |
| Potassium (K+) mM. | 5.0 ± 0.1 | 4.4 ± 0.5 | 3.6–5.5 |
| Total carbon dioxide (tCO2) mM | 25.0 ± 1.4 | 25.67 ± 2.1 | 14.0–26.0 |
| Chloride (CL−) (mM) | 108.5 ± 5.0 | 103.7 ± 4.9 | 102.0–120.0 |
| Glucose (mg/dL) | 86.5 ± 16.3 | 73.7 ± 10.6 | 70.0–138.0 |
| Calcium (Ca) mg/dL | 10.8 ± 0.3 | 10.6 ± 0.8 | 8.9–11.4 |
| Blood urea nitrogen (mg/dL) | 15.0 ± 1.4 | 9.3 ± 1.5 | 6.0–25.0 |
| Creatinine (mg/dL) | 0.8 ± 0.0 | 0.7 ± 0.1 | 0.5–1.6 |
| Alkaline Phosphatase (U/L) | 25.5 ± 3.5 | 22.7 ± 7.6 | 5.0–131.0 |
| Alanine aminotransferase (U/L) | 44.0 ± 1.4 | 41.7 ± 11.9 | 12.0–118.0 |
| Aspartate aminotransferase (U/L) | 26.0 ± 2.8 | 30.0 ± 4.4 | 15.0–66.0 |
| Total bilirubin (mg/dL) | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.1–0.3 |
| Albumin (g/dL) | 1.4 ± 0.1 | 1.3 ± 0.1 | 2.7–4.4 |
| Total protein (g/dL) | 6.6 ± 0.6 | 6.6 ± 0.8 | 5.2–8.8 |
Table 3.
Pre- and Post-Treatment Body Weights of Hound Dogs
| Dog number | Preweight (kg) | Postweight (kg) | P value (post- vs. preweight) | |
|---|---|---|---|---|
| Treated | 1 | 35 | 37 | 0.37 |
| 2 | 27 | 26 | ||
| 3 | 27 | 33 | ||
| Control | 4 | 37 | 39 | 0.36 |
| 5 | 25 | 34 |
Discussion
CKLP1 was developed as a prodrug to overcome the inadequate water solubility of KATP channel openers, which our laboratory has defined as a new class of ocular hypotensive agents. Like its parent compound levcromakalim, CKLP1 lowers IOP in animal models at similar efficacy.14,16–19 In this study, CKLP1 was shown to significantly lower IOP over extended periods of time, with no effects on systemic blood pressure, in 2 large animal models, hound dogs and African green monkeys. Pharmacokinetic analysis indicates that CKLP1 is cleaved into levcromakalim at sufficient amounts that result in significant lowering of IOP in normotensive animal eyes. A detailed histologic analysis of ocular tissues and systemic organs and blood chemistries from CKLP1-treated hound dogs did not reveal any significant pathology, suggesting excellent tolerability of the drug.
We have previously reported that CKLP1 lowers IOP by ∼17% in mice and 16% in Dutch-belted pigmented rabbits.18,19 However, we had not evaluated CKLP1 in a large animal or nonhuman primate model. In this study, we found that CKLP1 lowered IOP by ∼19% and 17% in hound dogs and African green monkeys, respectively. The trend of observing 16–19% IOP reduction in normotensive animals is consistent between small and large animals.14,16–19 One difference between this study and our previous studies is that maximal IOP reduction took 48–72 h in hound dogs and African green monkeys, slightly longer than previous results in mice and rabbits (<48 h). This could be because of reduced corneal penetration owing to thicker corneas in large animals. Average corneal thickness in hound dogs (550 μm) and monkeys (450 μm) is slightly thicker than Dutch-belted pigmented rabbits (350 μm) and nearly 5 times thicker than mice (100 μm).21–23 In addition, it is possible that conversion of CKLP1 to levcromakalim may occur at different rates among the species owing to underlying physiological differences, affecting the overall concentration of the active moiety. However, detailed tissue conversion analysis in different species will be required to validate the actual cause. Regardless of the slight delay in maximal IOP reduction, the ability of CKLP1 to lower IOP in hound dogs and African green monkeys validates this prodrug of levcromakalim as a potential hypotensive agent that can be utilized in ocular hypertensive diseases. Because the chamber angle of higher monkeys like the vervets is anatomically similar to humans,24 successful lowering of IOP in African green monkeys provides added confidence toward success of CKLP1 in human trials.
This is the first study to evaluate the pharmacokinetic properties of CKLP1 in a large animal model. Although pharmacokinetic studies in African green monkeys would have been ideal, it was not possible to perform a prolonged dose–response study nor was it feasible to analyze the blood and tissues from these monkeys because of ethical and regulatory issues. Therefore, for the detailed pharmacologic profiling of CKLP1, we focused on the normotensive hound dog model. Hound dogs have been extensively used as an appropriate model for various eye diseases including glaucoma,25 and some ocular hypotensive drugs have been analyzed in normotensive hound dogs and retain their IOP lowering efficacy.26,27 In our study, CKLP1 treatment of hound dogs showed conversion to its parent compound levcromakalim as evidenced by the longer Tmax of levcromakalim (∼120 min) compared with CKLP1 (∼60 min). However, this study was not specifically designed to analyze the efficiency or the rate of conversion, so the 10% conversion value reported is an estimate based on the comparison of levcromakalim with CKLP1 concentrations in blood. Because IOP was used as the physiological end-point parameter to assess the treatment efficiency of CKLP1, it is evident that the 10 mM dose used for this study generated a sufficient level of levcromakalim, which lowered IOP to levels previously reported in normotensive small animal (rodent) models.14,16,19 The optimum concentration of CKLP1 for lowering pressure, based on the dose–response studies in hound dogs, was the same as used in previous studies performed in Dutch-belted pigmented rabbits.19,20 For the dose–response studies, we used both eyes of dogs and treated them with different doses to expedite the experiment. Because contralateral eyes are not strictly independent, we performed pairwise t-tests after ANOVA to account for dependence. As for contralateral effect of treatment, all our previous studies with CKLP1 in mouse and rabbits have shown no evidence of drug involvement with the opposite eye.18–20 In addition, the extended dose study on the dogs and the short-term study on the nonhuman primates reported here also validate the lack of contralateral effects.
Levcromakalim, the active moiety of CKLP1 has well-known vasodilatory properties and was developed by Beecham Pharmaceuticals in the 1980s as an antihypertensive agent.28–31 Because drugs applied as eye drops have the potential to make their way into the systemic circulation, it was necessary to evaluate the blood pressure in both model systems to determine if the topical concentration had an effect on systolic or diastolic blood pressure. In both hound dogs and African green monkeys, CKLP1 had no significant effect on either systolic or diastolic pressure. Although our treatment in African green monkeys was for only 7 days, hound dogs showed no effect on blood pressure after 61 consecutive days of once-daily CKLP1 treatment. This is most likely because of the low concentrations of levcromakalim found in plasma (1 ng/mL), which is much lower than the reported threshold of the drug needed to elicit a systemic effect on blood pressure.29,31,32 However, this low level of levcromakalim is still enough to exert a localized IOP lowering effect, potentially through dilation of the vessels in the distal outflow pathway. Therefore, the topical ocular application appears to convert enough CKLP1 to levcromakalim to induce IOP reduction but is not enough to have an effect on blood pressure.
The low concentrations of levcromakalim found in blood could also be because of tissues acting as reservoirs for CKLP1, first by storing and then slowly releasing the drug. Values of AUC, which indicates the amount of available drug, is 22.4-fold higher for CKLP1 than levcromakalim, indicating that CKLP1 may be stored and then slowly released over time. The fact that several ocular tissues show high concentrations of CKLP1 and levcromakalim, adds further credibility to this theory. One such tissue appears to be the trabecular meshwork/aqueous plexus, which contains the most CKLP1 and levcromakalim among the analyzed ocular tissues. It is reasonable to speculate that the high concentration of CKLP1 identified in the trabecular meshwork is acting as a reservoir for slow release of levcromakalim in clinically relevant concentrations. This could also partially explain the 24–48 h delay in IOP returning to baseline after cessation of treatment, which has been identified in small animal models14,19,20 and also reported in this study. Because the trabecular meshwork is immediately proximal to the distal outflow region, it would be an ideal location for CKLP1 to levcromakalim conversion to induce an effect on the distal outflow pathway.
There are a couple of potential limitations to our study. We only had representative data from 2 hound dogs for tissue concentration evaluation. The third hound dog showed minimal levels of CKLP1 and levcromakalim that were below the level of quantitation. Although we believe this is owing to an aberrant sample collection issue, we cannot rule out the impact of individual physiologies toward the drug. Studies that utilize a larger sample size will be required to sort out this discrepancy.
Another potential limitation is that the African green monkeys used in this study required sedation during drug administration and IOP measurements (14 mg/kg ketamine intramuscular injection). Anesthetics have been reported to affect KATP channel function, suggesting they may alter the activity of KATP openers such as CKLP1.33,34 IOP reduction has been implied for ketamine use in monkeys but the extent of the reduction by ketamine alone is unclear.35 In contrast, studies on pediatric patients concluded that ketamine at low doses did not have any clinically relevant effect on IOP.36 In this study, even if ketamine did lower IOP, it would have affected both the treated, control, and baseline values. Despite the possible confounding effect of anesthetics, CKLP1 was still able to lower IOP by ∼17% compared with baseline.
In summary, this study establishes that CKLP1 is converted to levcromakalim after topical application to the eyes of hound dogs, which results in significant lowering of IOP without any effect on systemic blood pressure. Detailed analysis of tissue histology in the hound dogs did not reveal any observable toxicity caused by the treatment, or any substantial changes in blood chemistries, indicating excellent tolerance in these animals. Likewise, CKLP1 lowered IOP in African green monkeys with no effect on blood pressure. CKLP1 has been shown to be an effective ocular hypotensive agent in C57BL/6J mice, Dutch-belted pigmented rabbits, and now in hound dogs and African green monkeys. Future studies to assess the IOP lowering ability of CKLP1 in ocular hypertensive models will further strengthen the drug's position as a potential anti-glaucoma therapeutic with possible induction into human clinical trials.
Supplementary Material
Acknowledgments
The authors acknowledge the Preclinical Translational Services, Wake Forest School of Medicine, for help with the nonhuman primate studies. The authors also acknowledge Heather DeLoid, DVM, Assistant Professor of Comparative Medicine and Plastic Surgery, and Kortne Hudick and Stacy Combs, research laboratory technicians, for their help in collecting data from the African green monkeys.
Author Disclosure Statement
M.P.F. holds patent rights for CKLP1. None of the other authors have any conflicts of interests.
Funding Information
NIH grants EY21727 (M.P.F.); Mayo Clinic Department of Ophthalmology grant (U.R.C), Mayo Foundation (U.R.C., M.P.F.); Wake Forest Primate Signature Program Grant (U.R.C. and M.P.F.); CTSA and P40 grant (UL1-TR001420 and P40-OD010965; Wake Forest School of Medicine).
Supplementary Material
References
- 1. Weinreb, R.N., Aung, T., and Medeiros, F.A.. The pathophysiology and treatment of glaucoma: A review. JAMA 311:1901–1911, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weinreb, R.N., and Khaw, P.T.. Primary open-angle glaucoma. Lancet. 363:1711–1720, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Camras, C.B., Hedman, K., Group USLS.. Rate of response to latanoprost or timolol in patients with ocular hypertension or glaucoma. J. Glaucoma. 12:466–469, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Scherer, W.J. A retrospective review of non-responders to latanoprost. J. Ocul. Pharmacol. Ther. 18:287–291, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Aydin Kurna, S., Acikgoz, S., Altun, A., Ozbay, N., Sengor, T., and Olcaysu, O.O.. The effects of topical antiglaucoma drugs as monotherapy on the ocular surface: A prospective study. J. Ophthalmol. 2014:460483, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diggory, P., and Franks, W.. Medical treatment of glaucoma—a reappraisal of the risks. Br. J. Ophthalmol. 80:85–89, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mandell, A.I., Bruce, L.A., and Khalifa, M.A.. Reduced cyclic myopia with pilocarpine gel. Ann. Ophthalmol. 20:133–135, 1988 [PubMed] [Google Scholar]
- 8. Mantravadi, A.V., and Vadhar, N.. Glaucoma. Prim. Care. 42:437–449, 2015 [DOI] [PubMed] [Google Scholar]
- 9. Nguyen, Q.H. Combination of brinzolamide and brimonidine for glaucoma and ocular hypertension: Critical appraisal and patient focus. Patient Prefer Adherence. 8:853–864, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roy Chowdhury, U., Hann, C.R., Stamer, W.D., and Fautsch, M.P.. Aqueous humor outflow: Dynamics and disease. Invest. Ophthalmol. Vis. Sci. 56:2993–3003, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wistrand, P.J., Stjernschantz, J., and Olsson, K.. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv. Ophthalmol. 41 Suppl 2:S129–S138, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Sugrue, M.F. The pharmacology of antiglaucoma drugs. Pharmacol. Ther. 43:91–138, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Chowdhury, U.R., Bahler, C.K., Hann, C.R., Chang, M., Resch, Z.T., Romero, M.F., and Fautsch, M.P.. ATP-sensitive potassium (KATP) channel activation decreases intraocular pressure in the anterior chamber of the eye. Invest. Ophthalmol. Vis. Sci. 52:6435–6442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roy Chowdhury, U., Bahler, C.K., Holman, B.H., Dosa, P.I., and Fautsch, M.P.. Ocular hypotensive effects of the ATP-sensitive potassium channel opener cromakalim in human and murine experimental model systems. PLoS One. 10:e0141783, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roy Chowdhury, U., Bahler, C.K., Holman, B.H., and Fautsch, M.P.. ATP-sensitive potassium (KATP) channel openers diazoxide and nicorandil lower intraocular pressure by activating the Erk1/2 signaling pathway. PLoS One. 12:e0179345, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roy Chowdhury, U., Dosa, P.I., and Fautsch, M.P.. ATP sensitive potassium channel openers: A new class of ocular hypotensive agents. Exp. Eye Res. 158:85–93, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roy Chowdhury, U., Dosa, P.I., and Fautsch, M.P. Modulation of Intraocular pressure by ATP sensitive potassium channel openers. In: Samples, J.R., Knepper, P.A., eds. Glaucoma Research and Clinical Advances 2018 to 2020. Amsterdam, the Netherlands: Kugler Publications; 2018; 201–219 [Google Scholar]
- 18. Roy Chowdhury, U., Viker, K.B., Stoltz, K.L., Holman, B.H., Fautsch, M.P., and Dosa, P.I.. Analogs of the ATP-sensitive potassium (KATP) channel opener cromakalim with in vivo ocular hypotensive activity. J. Med. Chem. 59:6221–6231, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roy Chowdhury, U., Rinkoski, T.A., Bahler, C.K., Millar, J.C., Bertrand, J.A., Holman, B.H., Sherwood, J.M., Overby, D.R., Stoltz, K.L., Dosa, P.I., and Fautsch, M.P.. Effect of Cromakalim Prodrug 1 (CKLP1) on aqueous humor dynamics and feasibility of combination therapy with existing ocular hypotensive agents. Invest. Ophthalmol. Vis. Sci. 58:5731–5742, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roy Chowdhury, U., Kudgus, R.A., Rinkoski, T.A., Holman, B.H., Bahler, C.K., Hann, C.R., Reid, J.M., Dosa, P.I., and Fautsch, M.P.. Pharmacological and pharmacokinetic profile of the novel ocular hypotensive prodrug CKLP1 in Dutch-belted pigmented rabbits. PLoS One. 15:e0231841, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alario, A.F., and Pirie, C.G.. Central corneal thickness measurements in normal dogs: A comparison between ultrasound pachymetry and optical coherence tomography. Vet. Ophthalmol. 17:207–211, 2014 [DOI] [PubMed] [Google Scholar]
- 22. Elsmo, E.J., Kiland, J.A., Kaufman, P.L., and McLellan, G.J.. Evaluation of rebound tonometry in non-human primates. Exp. Eye Res. 92:268–273, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gandhi, J.K., Roy Chowdhury, U., Manzar, Z., Buck, J., Levin, L.R., Fautsch, M.P., and Marmorstein, A.D.. Differential Intraocular Pressure Measurements by Tonometry and Direct Cannulation After Treatment with Soluble Adenylyl Cyclase Inhibitors. J. Ocul. Pharmacol. Ther. 33:574–581, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barany E. Applanation Tonometry and Ophthalmoscopy of the Vervet Monkey (Cercopithecus Ethiops) in Phencyclidine Catalepsia. Invest. Ophthalmol. 2:322–324, 1963 [PubMed] [Google Scholar]
- 25. Bouhenni, R.A., Dunmire, J., Sewell, A., and Edward, D.P.. Animal models of glaucoma. J. Biomed. Biotechnol. 2012:692609, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gelatt, K.N., Gum, G.G., Gwin, R.M., Bromberg, N.M., Merideth, R.E., and Samuelson, D.A.. Primary open angle glaucoma: Inherited primary open angle glaucoma in the beagle. Am. J. Pathol. 102:292–295, 1981 [PMC free article] [PubMed] [Google Scholar]
- 27. Smith, L.N., Miller, P.E., and Felchle, L.M.. Effects of topical administration of latanoprost, timolol, or a combination of latanoprost and timolol on intraocular pressure, pupil size, and heart rate in clinically normal dogs. Am. J. Vet. Res. 71:1055–1061, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Buckingham, R.E., Clapham, J.C., Hamilton, T.C., Longman, S.D., Norton, J., and Poyser, R.H.. BRL 34915, a novel antihypertensive agent: Comparison of effects on blood pressure and other haemodynamic parameters with those of nifedipine in animal models. J. Cardiovasc. Pharmacol. 8:798–804, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Hamilton, T.C., and Weston, A.H.. Cromakalim, nicorandil and pinacidil: Novel drugs which open potassium channels in smooth muscle. Gen. Pharmacol. 20:1–9, 1989 [DOI] [PubMed] [Google Scholar]
- 30. Coldwell, M.C., and Howlett, D.R.. Specificity of action of the novel antihypertensive agent, BRL 34915, as a potassium channel activator. Comparison with nicorandil. Biochem. Pharmacol. 36:3663–3669, 1987 [DOI] [PubMed] [Google Scholar]
- 31. Hamilton, T.C., Beerahee, A., Moen, J.S., Price, R.K., Ramji, J.V., and Clapham, J.C.. Levcromakalim. Cardiovasc. Drug Rev. 11:199–222, 1993 [Google Scholar]
- 32. Wilson, C., Coldwell, M.C., Howlett, D.R., Cooper, S.M., and Hamilton, T.C.. Comparative effects of K+ channel blockade on the vasorelaxant activity of cromakalim, pinacidil and nicorandil. Eur. J. Pharmacol. 152:331–339, 1988 [DOI] [PubMed] [Google Scholar]
- 33. Kawano, T., Tanaka, K., Yinhua, Eguchi, S., Kawano, H., Takahashi, A., Nakaya, Y., and Oshita, S.. Effects of ketamine on nicorandil induced ATP-sensitive potassium channel activity in cell line derived from rat aortic smooth muscle. J. Med. Invest. 57:237–244, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Walsh, R.S., Tsuchida, A., Daly, J.J., Thornton, J.D., Cohen, M.V., and Downey, J.M.. Ketamine-xylazine anaesthesia permits a KATP channel antagonist to attenuate preconditioning in rabbit myocardium. Cardiovasc. Res. 28:1337–1341, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Jasien, J.V., Girkin, C.A., and Downs, J.C.. Effect of Anesthesia on Intraocular Pressure Measured With Continuous Wireless Telemetry in Nonhuman Primates. Invest. Ophthalmol. Vis. Sci. 60:3830–3834, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drayna, P.C., Estrada, C., Wang, W., Saville, B.R., and Arnold, D.H.. Ketamine sedation is not associated with clinically meaningful elevation of intraocular pressure. Am. J. Emerg. Med. 30:1215–1218, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.