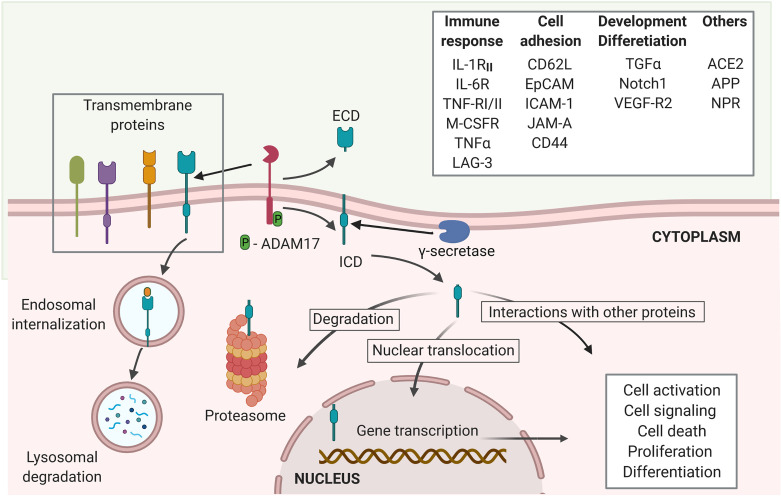
Figure 1.
Regulated intramembrane proteolysis process and proteins cleaved by ADAM17. Several proteins are targets of ADAM17 cleavage, including proteins involved in immune responses, cell adhesion and cellular development and differentiation, among others. The proteolytic activity of ADAM17 can be triggered through its phosphorylation by PKL2, PKC, and MAPKs. Activated ADAM17 cleaves several transmembrane proteins leading to the release of an extracellular domain (ECD) and the membrane retention of an intracellular cytoplasmic domain (ICD). The γ-secretase multi-protein complex removes this domain from the membrane allowing it to migrate to the cytoplasm where it can either be degraded at the proteasome or interact with other cytoplasmic proteins. Moreover, the ICD can trans-locate into the nuclei to induce transcriptional expression of several genes related to cell activation, signaling, death, proliferation and differentiation (created with BioRender.com).