Figure 3: and as a function of oxygen fugacity and carbon content in the alloy melt.
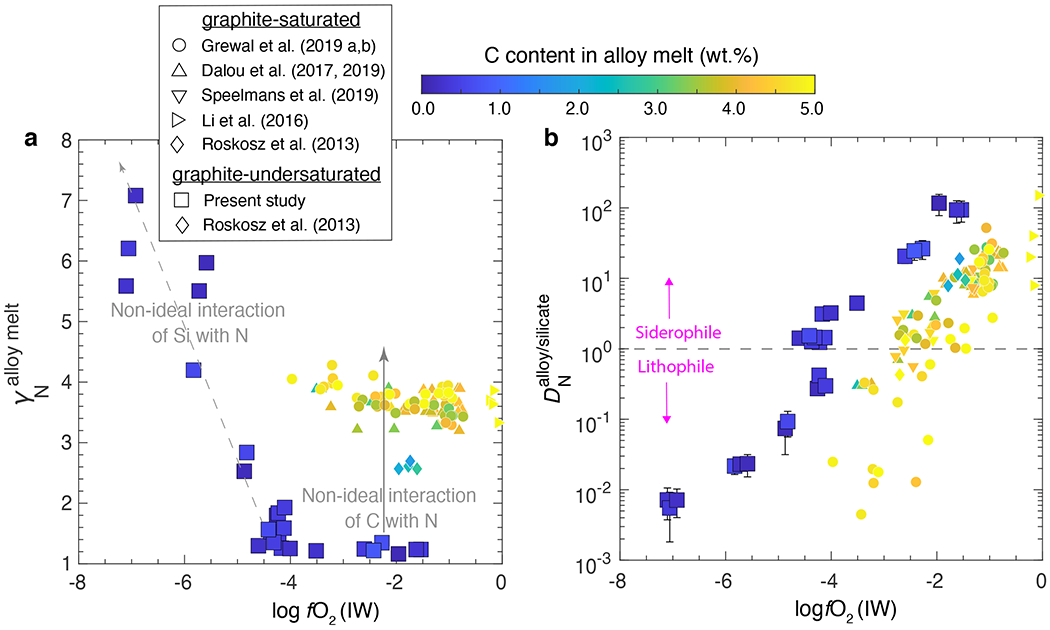
a) in C-poor and Si-free alloy melts in this study is ~1 while is ~3-4 at similar fO2 for graphite-saturated alloys. This is due to non-ideal interactions between C and N occupying similar octahedral voids in the alloy melt. Higher for C-poor and Si-bearing alloys results from repulsive interactions between Si and N. b) In agreement with previous studies in graphite-saturated conditions10–13,25,29, decreases with decreasing fO2 in graphite-undersaturated conditions. At any given fO2, in graphite-undersaturated conditions is almost an order of magnitude higher relative to graphite-saturated conditions. was calculated using the ‘Online Metal Activity Calculator’ (http://norris.org.au/expet/metalact/) which uses ε approach via Wagner equations. Error bars for represent ±1-σ deviation obtained by propagation of ±1-σ deviation on N content in the alloy and silicate melts; where absent, the error bars are smaller than the symbol size.
