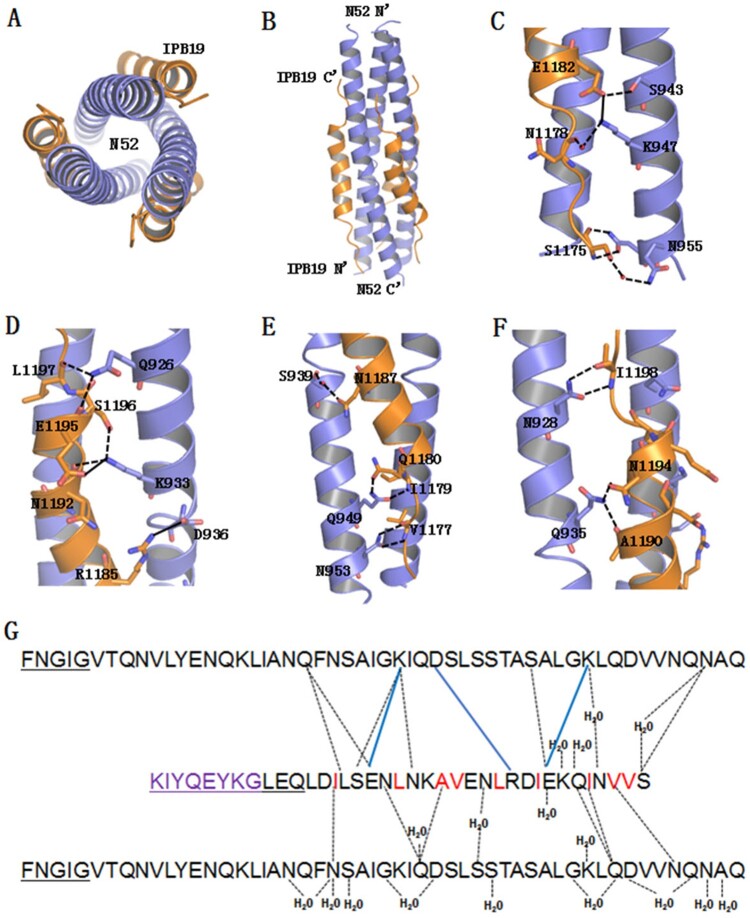
Figure 8.
Crystal structure of IPB19 complexed with N52. (A and B) The overall structure of IPB19/N52-based six-helical bundle (6-HB) is shown in a ribbon model and viewed from the bottom (A) or front (B) side, in which N52 helices (blue) form an interior, trimeric coiled coil with three hydrophobic grooves, three IPB19 helices (orange) pack into each of the grooves in an antiparallel orientation. (C-F) Detailed interactions between IPB19 and N52. The residues critical for the interactions are shown in sticks and labelled. The solid lines represent salt bridges, the dashed lines represent hydrogen bonds. (G) Sequence illustration of IPB19 binding. A single IPB19 peptide interacting with two N52 helices is shown in a sequence map. The blue solid lines represent salt bridges, the black dashed lines represent hydrogen bonds, and residues marked in red represent hydrophobic interactions. The residues in the C-terminal of IPB19 and the N-terminal of N52 that could not be visualized due to too low electronic density are underlined.