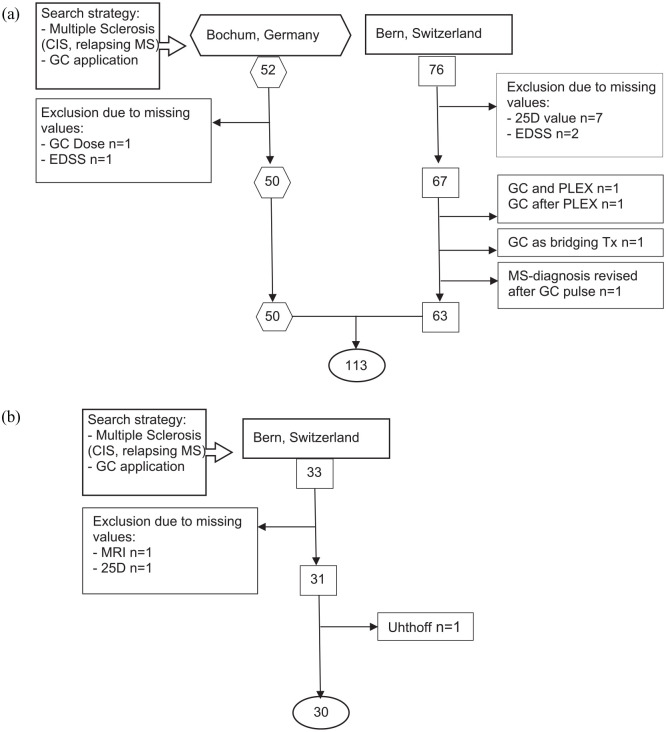
Figure 1.
Flowchart of the cohort formation process. We screened outpatient wards. (a) For the explorative cohort in Bochum from March 2013 until August 2015 (employment period of co-author at the Hospital of Bochum) and in Bern from 2014 (beginning of specialized neuroimmunological consultation) until March 2019. (b) For the validation cohort in Bern from March 2019 until December 2019. Screening terms were as listed above. We included any type of MS except progressive forms. Only 1 secondary progressive MS patient was included in Bern, because he had a clear relapse including MRI-enhancing lesions. By adding GC application, we restricted all found MS patients to those with acute relapse symptoms. If any needed value (GC dose, MRI, EDSS, 25D) could not be retrieved, patients were excluded from the analysis.
25D, 25-hydroxy-vitamin D; CIS, clinically isolated syndrome; EDSS, expanded disability status scale; GC, glucocorticoids; MS, multiple sclerosis; PLEX, plasma exchange; Tx, therapy.