Fig. 4: Predominate VSCC composition coincides with the morphology and function of osteoblasts and osteocytes.
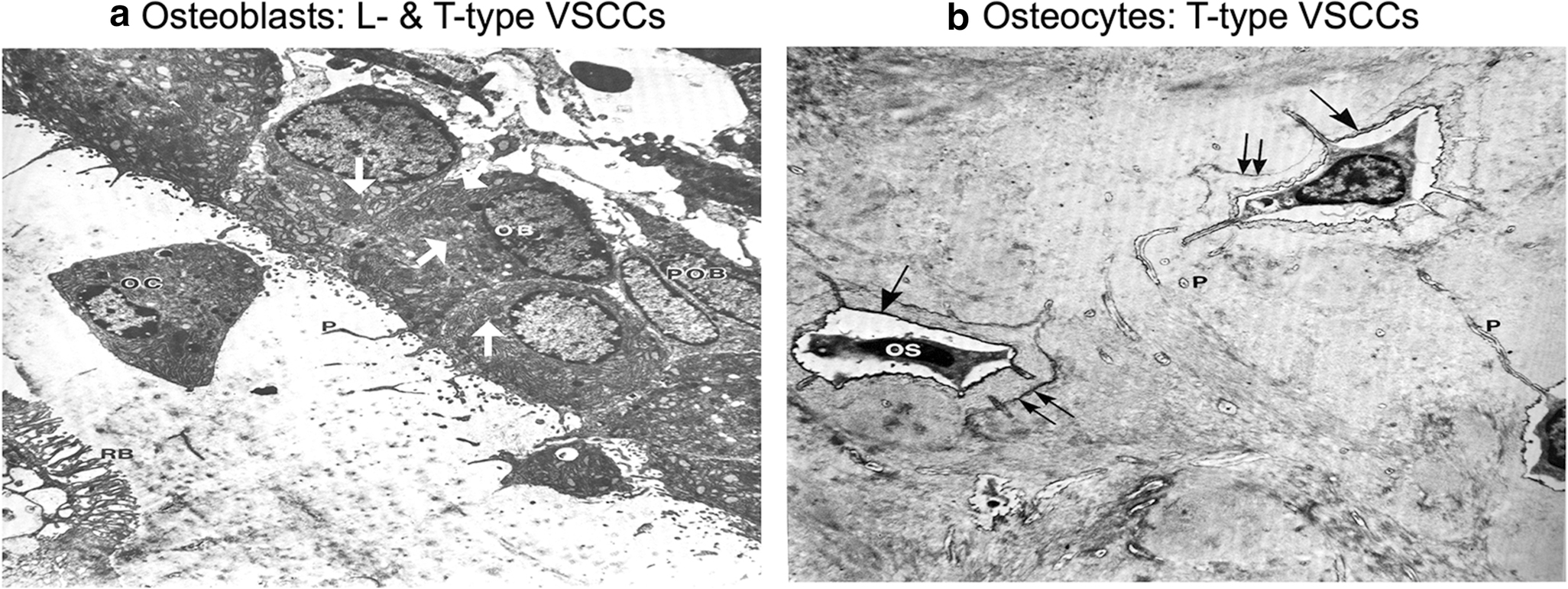
A). The enlarged ER and golgi secretory organelles (white arrows) of osteoblasts (OB) gives its characteristic “plump”, cuboidal morphology and high output of extracellular matrix. The predominate CaV1.2 L-type VSCCs in osteoblasts have a large single channel conductance and slow voltage-dependent inactivation, allowing for „long-lasting‟ calcium influx, the full release of ER calcium stores, and the stimulus necessary for bone formation and remodeling. B) With its flat, stellate shape, mature osteocytes (OS) have much smaller secretory organelles in comparison to osteoblasts. By shifting from expression of L-type to T-type VSCCs during differentiation, the presence of CaV3.2 VSCCs in osteocytes enables faster and more complete channel inactivation than L-type VSCCs, allowing osteocytes to retain its sensitivity to calcium influx without generating cytotoxic Ca2+ levels. Figures adapted from Butler TW [142].
