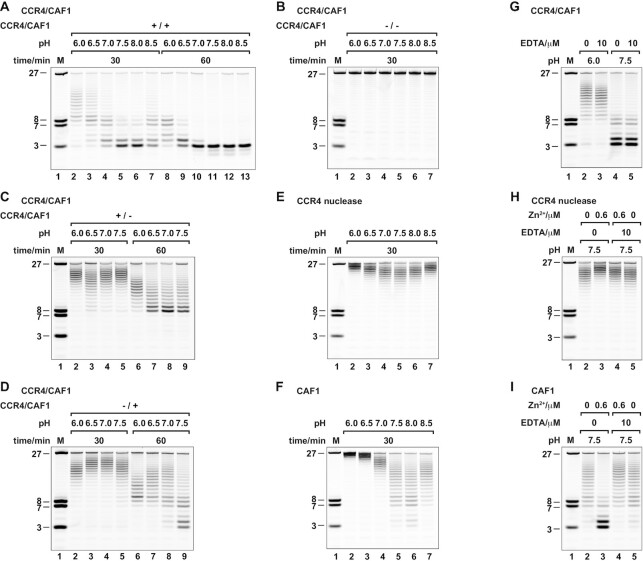
Figure 4.
Differential modulation of CCR4 and CAF1 nuclease activity by pH and zinc ions in stoichiometric amounts. (A, B) pH-dependence of the CCR4-CAF1 complex. Deadenylation occurs over a wide range of pH (A) and critically depends on the integrity of the active sites (B). (C, D) pH-dependence of the individual nucleases within the CCR4-CAF1 complex. Hydrolysis by CCR4 (+/−) occurs over a much narrower pH range than observed in panel (A) with an optimum at neutral to slightly acidic pH (C). Hydrolysis by CAF1 (−/+) shows a complex pH-dependence with apparently two pH optima at moderately acidic and moderately basic pH (D). (E, F) pH-dependence of the isolated individual CCR4 and CAF1 nucleases. Hydrolysis by the isolated CCR4 nuclease domain shows a single pH optimum at around neutral pH (E). Hydrolysis by the isolated CAF1 shows a single pH optimum at moderately basic pH (F), lacking the activity at moderately acidic pH observed in the context of inactivated CCR4 (D). Compare also Wang et al. (29) and Bianchin et al. (86) for initial characterizations of isolated human CCR4 nuclease (pH = 7.4) and isolated CAF1 (pH = 8.0), respectively. (G–I) Specific stimulation of CAF1 activity by stoichiometric quantities of zinc ion. Standard reaction conditions (containing 2 mM of magnesium ion) are not affected in the presence of 10 μM EDTA, used here as a strong zinc ion chelator (G, control). The isolated CCR4 nuclease domain (0.6 μM) is slightly inhibited by the addition of stoichiometric amounts of zinc ion (0.6 μM) to standard reaction conditions (H). The isolated CAF1 nuclease is clearly stimulated under the same conditions (I). DTT was omitted from (H) and (I) to avoid interference from its capacity to also act as a zinc ion chelator (see also Supplementary Figure S6).