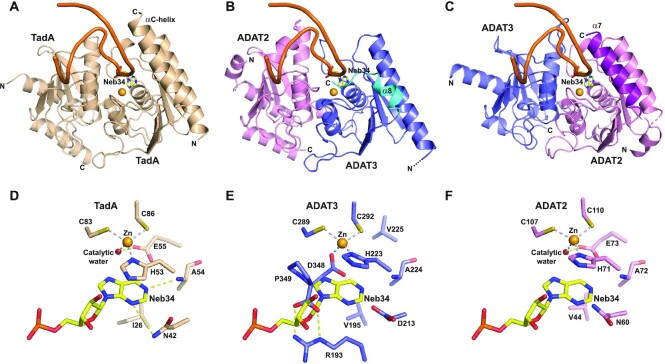
Figure 4.
ADAT2 but not ADAT3 can accommodate an anticodon stem-loop in its active site. (A) Structure of an anticodon-stem-loop (ASL) bound to S. aureus TadA (PDB code: 2b3j) (17). The ASL is shown as an orange ribbon and the catalytic zinc as a light orange sphere. The non-hydrolysable adenine analog, nebularine, at the wobble position is shown as sticks and labeled (Neb34). The αC-helix of TadA is required for ASL binding. (B) Model based on our structures and the structure shown in (A) of an ASL binding to mouse ADAT in the ADAT3 zinc-binding pocket. The shorter α8 helix and the position of the ADAT3 C-terminus are incompatible with the ASL binding. (C) Same as in (B) but with the ASL binding in the ADAT2 active site. The ASL and the wobble adenosine could be recognized, provided minor conformational rearrangements of the ADAT complex. ADAT2 long α7 helix could participate in ASL binding. (D) Close-up from (A) of the interaction of wobble Nebularine within TadA. The zinc and catalytic water are shown as light orange and red spheres, respectively. (E) Close-up from (B) of the putative interaction of wobble Nebularine within ADAT3 zinc-binding pocket. Binding is incompatible with the position of ADAT3 C-terminus, notably aspartate 348 and C-terminal proline 349. (F) Close-up from (C) of the putative interaction of wobble Nebularine within ADAT2 active site. TadA and ADAT2 share the same recognition determinants of the wobble base.